Synthesis ( IF 2.2 ) Pub Date : 2021-11-11 , DOI: 10.1055/a-1695-1095 Ivo Dias 1 , Sara C. Silva-Reis 1 , Beatriz L. Pires-Lima 1 , Xavier C. Correia 1 , Hugo F. Costa-Almeida 1
|
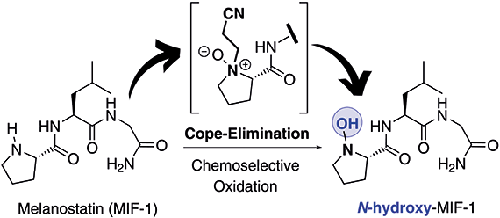
A convenient synthetic protocol for the unprecedented N-hydroxylation of proline residue in Melanostatin (MIF-1) neuropeptide is reported. This methodology is grounded on the incorporation of N-(cyanoethyl)prolyl residue followed by on-site oxidation by Cope elimination with m-chloroperbenzoic acid, exploring the unrecognized dual role of the cyanoethyl group as an effective N-protecting group under peptide synthesis conditions and as a suitable leaving group during the chemoselective on-site N-oxidation. Following this protocol N-hydroxy-MIF-1 is obtained in 78% global yield from N-(cyanoethyl)-
中文翻译:

使用 Cope 消除法对黑色素抑制素神经肽进行 N-羟基化的便捷现场氧化策略
报道了一种方便的合成方案,用于对黑素抑制素 (MIF-1) 神经肽中脯氨酸残基进行前所未有的N-羟基化。该方法基于引入N- (氰乙基)脯氨酸残基,然后通过用间氯过苯甲酸进行 Cope 消除进行现场氧化,探索了在肽合成条件下氰乙基作为有效N-保护基团的未被认识的双重作用并在化学选择性现场N-氧化过程中作为合适的离去基团。按照该协议, N-羟基-MIF-1 以 78% 的全球收率从N- (氰乙基)-中获得












































 京公网安备 11010802027423号
京公网安备 11010802027423号