Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-02-03 , DOI: 10.1016/j.bmcl.2022.128602 Meiyang Xi 1 , Chengjie Feng 2 , Kui Du 1 , Weiping Lv 3 , Chenxi Du 3 , Runpu Shen 2 , Haopeng Sun 3
|
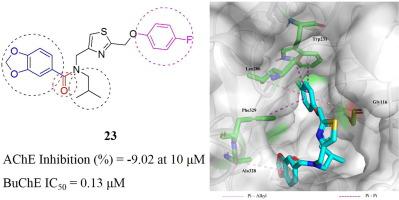
Butyrylcholinesterase (BuChE) is recently regarded as a biomarker in progressed Alzheimer’s disease (AD). Development of selective BuChE inhibitors has attracted a great deal of interest and may be a viable therapeutic strategy for AD. Recently, we reported the N-isobutyl-N-((2-(p-tolyloxymethyl)thiazol-4-yl)methyl)benzo[d][1,3]dioxole-5-carboxamide (1) as a selective BuChE inhibitor. Subsequently, 33 analogs were synthesized and assessed by AChE/BuChE activities, indicating an optimal compound 23. Further kinetic tests suggested a competitive manner. Molecular docking and Molecular dynamics (MD) simulation showed that it interacted with several residues in active site gorge of BuChE, possibly contributing to its selectivity and competitive pattern. Moreover, it showed low cytotoxicity and high blood brain barrier (BBB) permeability. Taken together, 23 was a promising BuChE inhibitor for the treatment of AD.
中文翻译:

N-异丁基-N-((2-(p-tolyloxymethyl)thiazol-4yl)methyl)benzo[d][1,3] dioxole-5-carboxamides 作为选择性丁酰胆碱酯酶抑制剂的设计、合成、生物学评价和分子模型
丁酰胆碱酯酶 (BuChE) 最近被认为是进展性阿尔茨海默病 (AD) 的生物标志物。选择性 BuChE 抑制剂的开发引起了极大的兴趣,并且可能是 AD 的可行治疗策略。最近,我们报道了N-异丁基-N -((2-(p-tolyloxymethyl)thiazol-4-yl)methyl)benzo[ d ][1,3]dioxole-5-carboxamide ( 1 ) 作为选择性 BuChE 抑制剂. 随后,合成了 33 种类似物并通过 AChE/BuChE 活性进行评估,表明最佳化合物23. 进一步的动力学测试表明了一种竞争方式。分子对接和分子动力学 (MD) 模拟表明,它与 BuChE 活性位点峡谷中的几个残基相互作用,可能有助于其选择性和竞争模式。此外,它显示出低细胞毒性和高血脑屏障(BBB)渗透性。总之,23是一种很有前途的 BuChE 抑制剂,可用于治疗 AD。































 京公网安备 11010802027423号
京公网安备 11010802027423号