Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-02-02 , DOI: 10.1016/j.molstruc.2022.132539 Jinyu Chang 1, 2 , Yuan Yao 3 , Yangfeng Xia 4 , Long Liu 2 , Yanqiang Zhang 2, 3
|
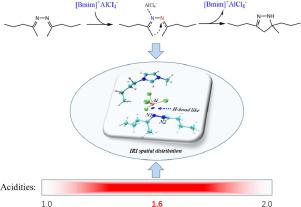
5-methyl-3,5-dipropyl-2-pyrazoline is an intermediate for the preparation of new high energy fuels. In this paper, chloroaluminate ionic liquids ([Bmim]Cl-xAlCl3, x = 1, 1.2, 1.4, 1.6, 1.8, 2.0) were applied as green catalysts for the synthesis of 5-methyl-3,5-dipropyl-2-pyrazoline through the intramolecular cyclization reaction of 2-pentyl ketazine. The effects of temperature, acidity and dosage of ionic liquids on the cyclization reaction were studied in detail and the highest catalytic performance was under the conditions as 110 °C, [Bmim]Cl-1.6AlCl3 and 10% of 2-pentyl ketazine. With the theoretical simulations, the possible catalytic mechanism was proposed as the hydrogen-like interaction between Al ([Bmim]+AlCl4−) and N1 atoms, which increases the charge of N2 atoms, thereby improving its nucleophilicity and further making it undergo intramolecular addition reaction with methyl. This study will provide guidance for the green preparation of 5-methyl-3,5-dipropyl-2-pyrazoline.
中文翻译:

氯铝酸盐离子液体催化制备5-甲基-3,5-二丙基-2-吡唑啉
5-甲基-3,5-二丙基-2-吡唑啉是制备新型高能燃料的中间体。本文将氯铝酸盐离子液体([Bmim]Cl-xAlCl 3 , x = 1, 1.2, 1.4, 1.6, 1.8, 2.0)作为绿色催化剂用于合成5-甲基-3,5-二丙基-2 -吡唑啉通过2-戊基酮嗪的分子内环化反应。详细研究了温度、酸度和离子液体用量对环化反应的影响,在110 ℃、[Bmim]Cl-1.6AlCl 3和10% 2-戊基酮嗪的条件下催化性能最高。通过理论模拟,提出了可能的催化机制为Al([Bmim] +AlCl 4 - ) 和N1原子,增加N2原子的电荷,从而提高其亲核性,并进一步使其与甲基发生分子内加成反应。本研究将为5-甲基-3,5-二丙基-2-吡唑啉的绿色制备提供指导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号