当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Labeling Cell Surface Receptors with Ligand.BirA* Bispecifics
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2022-02-01 , DOI: 10.1021/acsptsci.1c00192
Mays Alwash 1, 2 , Jean Gariépy 1, 2, 3
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2022-02-01 , DOI: 10.1021/acsptsci.1c00192
Mays Alwash 1, 2 , Jean Gariépy 1, 2, 3
Affiliation
|
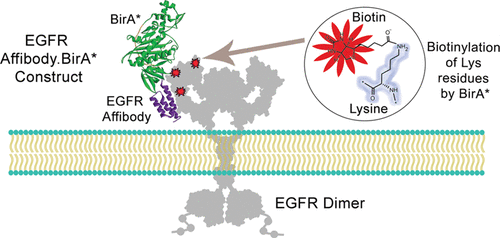
BirA*, a mutant form of the biotinylating enzyme BirA, can nonspecifically biotinylate ε-amino groups on lysines of proteins. Based on the promiscuous labeling nature of BirA*, plasmids expressing fusion constructs of BirA* to a given ligand have been used to transfect eukaryotic cells, leading to the biotinylation of intracellular proteins interacting or in close proximity to such Ligand.BirA* constructs. Mass spectrometry performed on the recovered biotinylated partners allows one to map intracellular protein interactors, a technique known as BioID. In contrast, the expression and purification of recombinant Ligand.BirA* constructs could serve as a powerful tool for labeling and detecting cell surface receptors. Here, we report the design and expression of recombinant Affibody.BirA* constructs, ZEGFR:1907.BirA* and ZHER2:243.BirA*, as protein bispecifics able to biotinylate their respective receptors EGFR and HER2 on the surface of MDA-MB-231 (EGFR+, EpCaM+, and CD44+) and SK-OV-3 (HER2++, EGFR+, EpCaM+, and CD44+) cancer cells. These Affibody.BirA* constructs retain both their BirA* enzymatic activity as well as their receptor-binding function. Importantly, MDA-MB-231 and SK-OV-3 cells biotinylated with Affibody.BirA* constructs did label their receptors EGFR and HER2 but did not biotinylate irrelevant antigens such as EpCaM or CD44 present on the surface of both cell lines. Ligand.BirA* bispecifics may represent a promising class of agents to identify unknown receptors on cell surfaces.
中文翻译:

用 Ligand.BirA* 双特异性标记细胞表面受体
BirA* 是生物素化酶 BirA 的一种突变形式,可以非特异性地生物素化蛋白质赖氨酸上的 ε-氨基。基于 BirA* 的混杂标记性质,表达 BirA* 与给定配体的融合构建体的质粒已被用于转染真核细胞,导致与这种 Ligand.BirA* 构建体相互作用或接近的细胞内蛋白质的生物素化。对回收的生物素化伙伴进行质谱分析可以绘制细胞内蛋白质相互作用物,这是一种称为 BioID 的技术。相比之下,重组 Ligand.BirA* 构建体的表达和纯化可以作为标记和检测细胞表面受体的有力工具。在这里,我们报告重组 Affibody.BirA* 构建体 Z EGFR:1907的设计和表达.BirA* 和 Z HER2:243 .BirA*,作为蛋白质双特异性蛋白,能够生物素化 MDA-MB-231(EGFR +、EpCaM +和 CD44 +)和 SK-OV-3表面上的各自受体 EGFR 和 HER2 (HER2 ++、EGFR +、EpCaM +和 CD44 +) 癌细胞。这些 Affibody.BirA* 构建体保留了它们的 BirA* 酶活性以及它们的受体结合功能。重要的是,用 Affibody.BirA* 构建体生物素化的 MDA-MB-231 和 SK-OV-3 细胞确实标记了它们的受体 EGFR 和 HER2,但没有生物素化两种细胞系表面上存在的不相关抗原,例如 EpCaM 或 CD44。Ligand.BirA* 双特异性可能代表一类有前途的试剂来识别细胞表面上的未知受体。
更新日期:2022-02-11
中文翻译:

用 Ligand.BirA* 双特异性标记细胞表面受体
BirA* 是生物素化酶 BirA 的一种突变形式,可以非特异性地生物素化蛋白质赖氨酸上的 ε-氨基。基于 BirA* 的混杂标记性质,表达 BirA* 与给定配体的融合构建体的质粒已被用于转染真核细胞,导致与这种 Ligand.BirA* 构建体相互作用或接近的细胞内蛋白质的生物素化。对回收的生物素化伙伴进行质谱分析可以绘制细胞内蛋白质相互作用物,这是一种称为 BioID 的技术。相比之下,重组 Ligand.BirA* 构建体的表达和纯化可以作为标记和检测细胞表面受体的有力工具。在这里,我们报告重组 Affibody.BirA* 构建体 Z EGFR:1907的设计和表达.BirA* 和 Z HER2:243 .BirA*,作为蛋白质双特异性蛋白,能够生物素化 MDA-MB-231(EGFR +、EpCaM +和 CD44 +)和 SK-OV-3表面上的各自受体 EGFR 和 HER2 (HER2 ++、EGFR +、EpCaM +和 CD44 +) 癌细胞。这些 Affibody.BirA* 构建体保留了它们的 BirA* 酶活性以及它们的受体结合功能。重要的是,用 Affibody.BirA* 构建体生物素化的 MDA-MB-231 和 SK-OV-3 细胞确实标记了它们的受体 EGFR 和 HER2,但没有生物素化两种细胞系表面上存在的不相关抗原,例如 EpCaM 或 CD44。Ligand.BirA* 双特异性可能代表一类有前途的试剂来识别细胞表面上的未知受体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号