当前位置:
X-MOL 学术
›
ACS Appl. Polym. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Impact of Diethyl Furan-2,5-dicarboxylate as an Aromatic Biobased Monomer toward Lipase-Catalyzed Synthesis of Semiaromatic Copolyesters
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2022-02-01 , DOI: 10.1021/acsapm.1c01777
Kifah Nasr 1, 2 , Audrey Favrelle-Huret 1 , Rosica Mincheva 2 , Gregory Stoclet 3 , Marc Bria 4 , Jean-Marie Raquez 2 , Philippe Zinck 1
ACS Applied Polymer Materials ( IF 4.4 ) Pub Date : 2022-02-01 , DOI: 10.1021/acsapm.1c01777
Kifah Nasr 1, 2 , Audrey Favrelle-Huret 1 , Rosica Mincheva 2 , Gregory Stoclet 3 , Marc Bria 4 , Jean-Marie Raquez 2 , Philippe Zinck 1
Affiliation
|
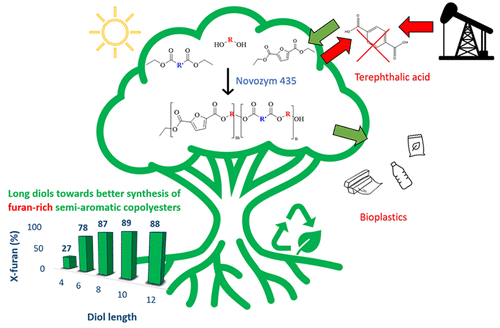
Furan-2,5-dicarboxylic acid has been introduced in recent years as a green aromatic monomer toward the design of aromatic (co)polyesters with enhanced properties, i.e., polyethylene furanoate (PEF) that can definitely compete with its petroleum-based counterpart, i.e., polyethylene terephthalate (PET). In an attempt to produce biobased semiaromatic copolyesters in an efficient eco-friendly approach, we report herein the polycondensation of diethyl furan-2,5-dicarboxylate (DEFDC) with different aliphatic diols and diesters of variable chain length catalyzed by an immobilized lipase from Candida antarctica using a two-step polymerization reaction carried out in diphenyl ether. The influence of diol and diester chain length, the molar concentration of DEFDC, and the effect of enzyme loading were assessed via nuclear magnetic resonance (NMR), gel permeation chromatography (GPC), differential scanning calorimetry (DSC), and wide-angle X-ray scattering (WAXS). With high quantities of DEFDC, significant differences in terms of M̅n buildup were noticed. Only longer diols starting from octane-1,8-diol successfully reacted with up to 90% DEFDC as opposed to only 25% DEFDC reacting with short diols such as butane-1,4-diol. While varying the chain length of the diester, it was evident that shorter diols such as hexane-1,6-diol have better reactivity toward longer diesters, while dodecane-1,12-diol was reactive toward all tested diesters. The incorporation of long chain fatty dimer diols such as Pripol 2033 led to polyesters with higher M̅n and was successfully used to overcome the limitations of poor reactivity observed in the case of short diols in the presence of high furan content. The DSC results showed a pseudoeutectic behavior as a function of increasing the mol % of DEFDC, and a change in the crystalline phase was confirmed via WAXS analysis. Finally, this work showed the successful enzyme-catalyzed synthesis of several DEFDC biobased semiaromatic copolyesters with variable interesting properties that can be further optimized for possible applications in food packaging as well as other possibilities.
中文翻译:

呋喃-2,5-二羧酸二乙酯作为芳香族生物基单体对脂肪酶催化合成半芳香族共聚酯的影响
近年来,呋喃-2,5-二羧酸作为一种绿色芳香族单体被引入,用于设计具有增强性能的芳香族(共)聚酯,即绝对可以与其石油基对应物竞争的聚呋喃甲酸乙二醇酯(PEF),即聚对苯二甲酸乙二醇酯(PET)。为了以一种有效的环保方法生产生物基半芳族共聚酯,我们在此报告了由念珠菌固定化脂肪酶催化的 2,5-二羧酸二乙酯 (DEFDC) 与不同链长的不同脂肪族二醇和二酯的缩聚反应。南极洲使用在二苯醚中进行的两步聚合反应。通过核磁共振 (NMR)、凝胶渗透色谱法 (GPC)、差示扫描量热法 (DSC) 和广角 X 评估二醇和二酯链长、DEFDC 摩尔浓度和酶负载的影响-射线散射(WAXS)。使用大量 DEFDC,M̅ n方面的显着差异注意到堆积。只有从 1,8-辛烷二醇开始的较长二醇成功地与高达 90% 的 DEFDC 反应,而只有 25% 的 DEFDC 与短二醇如 1,4-丁二醇反应。虽然改变二酯的链长,但很明显较短的二醇如 1,6-己二醇对较长的二酯具有更好的反应性,而十二烷-1,12-二醇对所有测试的二酯具有反应性。长链脂肪二聚二醇如 Pripol 2033 的加入导致聚酯具有更高的M̅ n并成功地用于克服在高呋喃含量存在下短二醇的情况下观察到的反应性差的局限性。DSC 结果显示随着 DEFDC 的 mol% 增加而产生的假共晶行为,并且通过 WAXS 分析证实了结晶相的变化。最后,这项工作展示了几种 DEFDC 生物基半芳族共聚酯的成功酶催化合成,这些聚酯具有可变的有趣特性,可以进一步优化用于食品包装的可能应用以及其他可能性。
更新日期:2022-02-11
中文翻译:

呋喃-2,5-二羧酸二乙酯作为芳香族生物基单体对脂肪酶催化合成半芳香族共聚酯的影响
近年来,呋喃-2,5-二羧酸作为一种绿色芳香族单体被引入,用于设计具有增强性能的芳香族(共)聚酯,即绝对可以与其石油基对应物竞争的聚呋喃甲酸乙二醇酯(PEF),即聚对苯二甲酸乙二醇酯(PET)。为了以一种有效的环保方法生产生物基半芳族共聚酯,我们在此报告了由念珠菌固定化脂肪酶催化的 2,5-二羧酸二乙酯 (DEFDC) 与不同链长的不同脂肪族二醇和二酯的缩聚反应。南极洲使用在二苯醚中进行的两步聚合反应。通过核磁共振 (NMR)、凝胶渗透色谱法 (GPC)、差示扫描量热法 (DSC) 和广角 X 评估二醇和二酯链长、DEFDC 摩尔浓度和酶负载的影响-射线散射(WAXS)。使用大量 DEFDC,M̅ n方面的显着差异注意到堆积。只有从 1,8-辛烷二醇开始的较长二醇成功地与高达 90% 的 DEFDC 反应,而只有 25% 的 DEFDC 与短二醇如 1,4-丁二醇反应。虽然改变二酯的链长,但很明显较短的二醇如 1,6-己二醇对较长的二酯具有更好的反应性,而十二烷-1,12-二醇对所有测试的二酯具有反应性。长链脂肪二聚二醇如 Pripol 2033 的加入导致聚酯具有更高的M̅ n并成功地用于克服在高呋喃含量存在下短二醇的情况下观察到的反应性差的局限性。DSC 结果显示随着 DEFDC 的 mol% 增加而产生的假共晶行为,并且通过 WAXS 分析证实了结晶相的变化。最后,这项工作展示了几种 DEFDC 生物基半芳族共聚酯的成功酶催化合成,这些聚酯具有可变的有趣特性,可以进一步优化用于食品包装的可能应用以及其他可能性。






































 京公网安备 11010802027423号
京公网安备 11010802027423号