当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthetic Studies of Aziridine Formation in Azicemicins
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-12-23 , DOI: 10.1021/ja907307h
Yasushi Ogasawara 1 , Hung-wen Liu
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-12-23 , DOI: 10.1021/ja907307h
Yasushi Ogasawara 1 , Hung-wen Liu
Affiliation
|
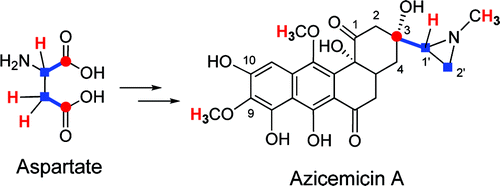
The azicemicins, which are angucycline-type antibiotics produced by the actinomycete, Kibdelosporangium sp. MJ126-NF4, contain an aziridine ring attached to the polyketide core. Feeding experiments using [1-(13)C]acetate or [1,2-(13)C(2)]acetate indicated that the angucycline skeleton is biosynthesized by a type II polyketide synthase. Isotope-tracer experiments using deuterium-labeled amino acids revealed that aspartic acid is the precursor of the aziridine moiety. Subsequent cloning and sequencing efforts led to the identification of the azicemicin (azic) gene cluster spanning approximately 50 kbp. The cluster harbors genes typical for type II polyketide synthesis. Also contained in the cluster are genes for two adenylyl transferases, a decarboxylase, an additional acyl carrier protein (ACP), and several oxygenases. On the basis of the assigned functions of these genes, a possible pathway for aziridine ring formation in the azecimicins can now be proposed. To obtain support for the proposed biosynthetic pathway, two genes encoding adenylyltransferases were overexpressed and the resulting proteins were purified. Enzyme assays showed that one of the adenylyltransferases specifically recognizes aspartic acid, providing strong evidence, in addition to the feeding experiments, that aspartate is the precursor of the aziridine moiety. The results reported herein set the stage for future biochemical studies of aziridine biosynthesis and assembly.
中文翻译:

阿齐西霉素中氮丙啶形成的生物合成研究
阿齐西霉素是由放线菌 Kibdelosporangium sp. 产生的angucycline 型抗生素。MJ126-NF4,包含一个连接到聚酮化合物核的氮丙啶环。使用 [1-(13)C] 乙酸盐或 [1,2-(13)C(2)] 乙酸盐的喂养实验表明,angucycline 骨架是由 II 型聚酮化合物合成酶生物合成的。使用氘标记的氨基酸的同位素示踪剂实验表明天冬氨酸是氮丙啶部分的前体。随后的克隆和测序工作导致鉴定了跨越大约 50 kbp 的阿齐霉素 (azic) 基因簇。该簇含有典型的 II 型聚酮化合物合成基因。该簇中还包含两种腺苷酸转移酶、一种脱羧酶、一种额外的酰基载体蛋白 (ACP) 和几种加氧酶的基因。根据这些基因的指定功能,现在可以提出在阿西霉素中形成氮丙啶环的可能途径。为了获得对提议的生物合成途径的支持,两个编码腺苷酸转移酶的基因被过表达,并且产生的蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。两种编码腺苷酸转移酶的基因被过表达,所得蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。两种编码腺苷酸转移酶的基因被过表达,所得蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。
更新日期:2009-12-23
中文翻译:

阿齐西霉素中氮丙啶形成的生物合成研究
阿齐西霉素是由放线菌 Kibdelosporangium sp. 产生的angucycline 型抗生素。MJ126-NF4,包含一个连接到聚酮化合物核的氮丙啶环。使用 [1-(13)C] 乙酸盐或 [1,2-(13)C(2)] 乙酸盐的喂养实验表明,angucycline 骨架是由 II 型聚酮化合物合成酶生物合成的。使用氘标记的氨基酸的同位素示踪剂实验表明天冬氨酸是氮丙啶部分的前体。随后的克隆和测序工作导致鉴定了跨越大约 50 kbp 的阿齐霉素 (azic) 基因簇。该簇含有典型的 II 型聚酮化合物合成基因。该簇中还包含两种腺苷酸转移酶、一种脱羧酶、一种额外的酰基载体蛋白 (ACP) 和几种加氧酶的基因。根据这些基因的指定功能,现在可以提出在阿西霉素中形成氮丙啶环的可能途径。为了获得对提议的生物合成途径的支持,两个编码腺苷酸转移酶的基因被过表达,并且产生的蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。两种编码腺苷酸转移酶的基因被过表达,所得蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。两种编码腺苷酸转移酶的基因被过表达,所得蛋白质被纯化。酶分析表明,其中一种腺苷酸转移酶特异性识别天冬氨酸,这提供了强有力的证据,除了喂养实验外,天冬氨酸是氮丙啶部分的前体。本文报告的结果为未来氮丙啶生物合成和组装的生化研究奠定了基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号