当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Free-radical catalyzed oxidation reactions with cyclohexene and cyclooctene with peroxides as initiators
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-02-01 , DOI: 10.1002/poc.4326 Rudy L. Luck 1 , Nick K. Newberry 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2022-02-01 , DOI: 10.1002/poc.4326 Rudy L. Luck 1 , Nick K. Newberry 1
Affiliation
|
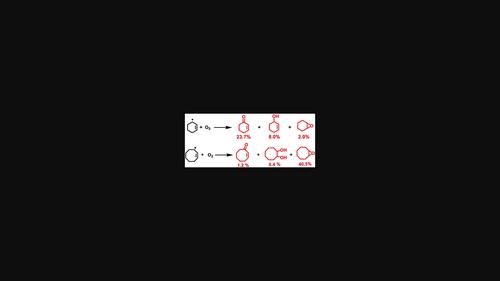
The free-radical oxidation reaction of cyclohexene is accomplished at 80°C in a 10:1 1,2-dichloroethane:acetonitrile mixture under an air atmosphere using 0.05 molar equivalents of either tBuOOH or H2O2 and results in a 33.7% conversion yielding 23.7% 2-cyclohexen-1-one, 8.0% 2-cyclohexen-1-ol, and 2.0% cyclohexene oxide. With cyclooctene under similar conditions, a 42.3% conversion is obtained, 40.3% cyclooctene oxide, 0.4% of 1,2-cyclooctanediol, and 1.2% of 2-cycloocten-1-one. The reaction is dependent on dioxygen as reduced yields are obtained under a nitrogen atmosphere. It is initiated best with H2O2 and tBuOOH and does not work with ammonium persulfate as a radical initiator. This free-radical reaction was not useful for three other alkenes tested under similar reaction conditions. Transition states and activation energies were calculated with the APFD functional and 6-311 + G(d) basis set for possible reaction pathways involving radicals produced from the homolysis of the peroxide OO bond followed by abstraction of an alkyl H atom, forming a cyclic alkene radical. This can react rapidly with dioxygen forming a cyclic-alkene peroxide radical. This intermediate may result in ketone products via a unimolecular pathway, whereas the formation of epoxides requires a bimolecular route. This was confirmed experimentally as lower epoxide yields for reaction with cyclooctene were obtained if the reaction solution was diluted. It is suggested that reactions involving cyclohexene where similar yields of products were obtained may also involve free-radical pathways.
中文翻译:

用过氧化物作为引发剂的环己烯和环辛烯的自由基催化氧化反应
环己烯的自由基氧化反应在 80°C 下在 10:1 1,2-二氯乙烷:乙腈混合物中在空气气氛下使用 0.05 摩尔当量的t BuOOH 或 H 2 O 2完成,产生 33.7%转化产生 23.7% 2-cyclohexen-1-one、8.0% 2-cyclohexen-1-ol 和 2.0% 氧化环己烯。在类似条件下使用环辛烯,得到 42.3% 的转化率、40.3% 的氧化环辛烯、0.4% 的 1,2-环辛二醇和 1.2% 的 2-cycloocten-1-one。该反应依赖于分子氧,因为在氮气氛下获得了降低的产率。最好用 H 2 O 2和tBuOOH 不能与过硫酸铵作为自由基引发剂一起使用。这种自由基反应对在类似反应条件下测试的其他三种烯烃没有用处。过渡态和活化能是用 APFD 泛函和 6-311 + G(d) 基组计算的,可能的反应途径涉及由过氧化物 O 的均裂产生的自由基O 键后脱去烷基 H 原子,形成环状烯烃自由基。这可以与分子氧快速反应,形成环状烯烃过氧化物自由基。该中间体可能通过单分子途径产生酮产物,而环氧化物的形成需要双分子途径。实验证实了这一点,因为如果稀释反应溶液,则与环辛烯反应的环氧化物产率较低。有人提出,在获得类似产率的产物的情况下,涉及环己烯的反应也可能涉及自由基途径。
更新日期:2022-02-01
中文翻译:

用过氧化物作为引发剂的环己烯和环辛烯的自由基催化氧化反应
环己烯的自由基氧化反应在 80°C 下在 10:1 1,2-二氯乙烷:乙腈混合物中在空气气氛下使用 0.05 摩尔当量的t BuOOH 或 H 2 O 2完成,产生 33.7%转化产生 23.7% 2-cyclohexen-1-one、8.0% 2-cyclohexen-1-ol 和 2.0% 氧化环己烯。在类似条件下使用环辛烯,得到 42.3% 的转化率、40.3% 的氧化环辛烯、0.4% 的 1,2-环辛二醇和 1.2% 的 2-cycloocten-1-one。该反应依赖于分子氧,因为在氮气氛下获得了降低的产率。最好用 H 2 O 2和tBuOOH 不能与过硫酸铵作为自由基引发剂一起使用。这种自由基反应对在类似反应条件下测试的其他三种烯烃没有用处。过渡态和活化能是用 APFD 泛函和 6-311 + G(d) 基组计算的,可能的反应途径涉及由过氧化物 O 的均裂产生的自由基O 键后脱去烷基 H 原子,形成环状烯烃自由基。这可以与分子氧快速反应,形成环状烯烃过氧化物自由基。该中间体可能通过单分子途径产生酮产物,而环氧化物的形成需要双分子途径。实验证实了这一点,因为如果稀释反应溶液,则与环辛烯反应的环氧化物产率较低。有人提出,在获得类似产率的产物的情况下,涉及环己烯的反应也可能涉及自由基途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号