当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Vitro Selection of Foldamer-Like Macrocyclic Peptides Containing 2-Aminobenzoic Acid and 3-Aminothiophene-2-Carboxylic Acid
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-01-31 , DOI: 10.1021/jacs.1c12133 Takayuki Katoh 1 , Hiroaki Suga 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-01-31 , DOI: 10.1021/jacs.1c12133 Takayuki Katoh 1 , Hiroaki Suga 1
Affiliation
|
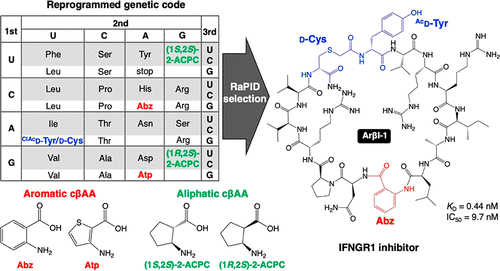
Aromatic cyclic β2,3-amino acids (cβAAs), such as 2-aminobenzoic acid and 3-aminothiophene-2-carboxylic acid, are building blocks that can induce unique folding propensities of peptides. Although their ribosomal elongation had been a formidable task due to the low nucleophilicity of their amino groups, we have recently overcome this issue by means of an engineered tRNAPro1E2 that enhances their incorporation efficiency into nascent peptide chains. Here we report ribosomal synthesis of a random macrocyclic peptide library containing aromatic and aliphatic cβAAs, and its application to de novo discovery of binders against human IFNGR1 and FXIIa as model targets. The potent binding peptides showed not only high inhibitory activity but also high protease resistance in human serum. Moreover, these cβAAs play a critical role in exhibiting their properties, establishing a discovery platform for de novo foldamer-like macrocycles containing such unique building blocks.
中文翻译:

含有2-氨基苯甲酸和3-氨基噻吩-2-羧酸的类折叠大环肽的体外选择
芳香环 β 2,3-氨基酸 (cβAAs),例如 2-氨基苯甲酸和 3-氨基噻吩-2-羧酸,是可以诱导肽的独特折叠倾向的构件。尽管由于其氨基的低亲核性,它们的核糖体延伸一直是一项艰巨的任务,但我们最近通过工程化的 tRNA Pro1E2克服了这个问题这提高了它们在新生肽链中的掺入效率。在这里,我们报告了含有芳香族和脂肪族 cβAAs 的随机大环肽库的核糖体合成,及其在从头发现针对人类 IFNGR1 和 FXIIa 的结合剂作为模型靶标的应用。强效结合肽在人血清中不仅表现出高抑制活性,而且表现出高蛋白酶抗性。此外,这些 cβAAs 在展示其特性方面发挥着关键作用,为含有这种独特构建块的从头折叠体状大环建立了一个发现平台。
更新日期:2022-02-09
中文翻译:

含有2-氨基苯甲酸和3-氨基噻吩-2-羧酸的类折叠大环肽的体外选择
芳香环 β 2,3-氨基酸 (cβAAs),例如 2-氨基苯甲酸和 3-氨基噻吩-2-羧酸,是可以诱导肽的独特折叠倾向的构件。尽管由于其氨基的低亲核性,它们的核糖体延伸一直是一项艰巨的任务,但我们最近通过工程化的 tRNA Pro1E2克服了这个问题这提高了它们在新生肽链中的掺入效率。在这里,我们报告了含有芳香族和脂肪族 cβAAs 的随机大环肽库的核糖体合成,及其在从头发现针对人类 IFNGR1 和 FXIIa 的结合剂作为模型靶标的应用。强效结合肽在人血清中不仅表现出高抑制活性,而且表现出高蛋白酶抗性。此外,这些 cβAAs 在展示其特性方面发挥着关键作用,为含有这种独特构建块的从头折叠体状大环建立了一个发现平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号