当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Axially Chiral Aldehydes by N-Heterocyclic-Carbene-Catalyzed Desymmetrization Followed by Kinetic Resolution
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-01-31 , DOI: 10.1002/anie.202117340 Yingtao Wu 1 , Mingrui Li 1 , Jiaqiong Sun 2 , Guangfan Zheng 1 , Qian Zhang 1, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-01-31 , DOI: 10.1002/anie.202117340 Yingtao Wu 1 , Mingrui Li 1 , Jiaqiong Sun 2 , Guangfan Zheng 1 , Qian Zhang 1, 3
Affiliation
|
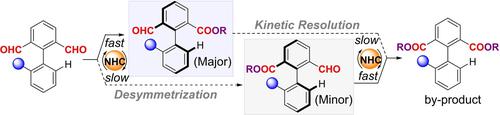
N-Heterocyclic carbene (NHC)-catalyzed atroposelective esterification of dialdehydes led to structurally diverse axially chiral aldehydes in good yields, with high enantioselectivity enabled by unprecedented NHC-catalyzed desymmetrization of the dialdehydes followed by kinetic resolution. This protocol, which features mild reaction conditions and broad functional-group tolerance, provides modular access to value-added axially chiral aldehydes and their derivatives.
中文翻译:

N-杂环卡宾催化的去对称化和动力学拆分合成轴向手性醛
N-杂环卡宾 (NHC) 催化的二醛的转位选择性酯化导致结构多样的轴向手性醛以良好的收率获得,通过前所未有的 NHC 催化的二醛去对称化和动力学拆分实现了高对映选择性。该协议具有温和的反应条件和广泛的官能团耐受性,提供了对增值轴向手性醛及其衍生物的模块化访问。
更新日期:2022-01-31
中文翻译:

N-杂环卡宾催化的去对称化和动力学拆分合成轴向手性醛
N-杂环卡宾 (NHC) 催化的二醛的转位选择性酯化导致结构多样的轴向手性醛以良好的收率获得,通过前所未有的 NHC 催化的二醛去对称化和动力学拆分实现了高对映选择性。该协议具有温和的反应条件和广泛的官能团耐受性,提供了对增值轴向手性醛及其衍生物的模块化访问。































 京公网安备 11010802027423号
京公网安备 11010802027423号