当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic MOF Nanoparticles Delivery of C-Dot Nanozyme and CRISPR/Cas9 System for Site-Specific Treatment of Ulcerative Colitis
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-01-31 , DOI: 10.1021/acsami.1c21700 Yana Ma 1, 2 , Wenhui Gao 1, 2 , Yujie Zhang 1, 2 , Mei Yang 1, 2 , Xiangji Yan 1, 2 , Yuanyuan Zhang 1, 2 , Guanying Li 1, 2 , Cui Liu 1, 2 , Changlong Xu 3 , Mingzhen Zhang 1, 2, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-01-31 , DOI: 10.1021/acsami.1c21700 Yana Ma 1, 2 , Wenhui Gao 1, 2 , Yujie Zhang 1, 2 , Mei Yang 1, 2 , Xiangji Yan 1, 2 , Yuanyuan Zhang 1, 2 , Guanying Li 1, 2 , Cui Liu 1, 2 , Changlong Xu 3 , Mingzhen Zhang 1, 2, 4
Affiliation
|
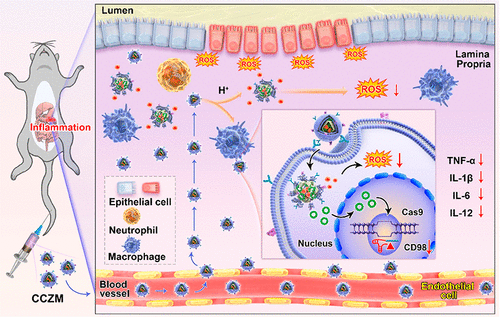
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) of unknown etiology affecting the colon and rectum. Previous studies have found that reactive oxygen species (ROS) overproduction and transmembrane glycoprotein CD98 (encoded by SLC3A2) upregulation played important roles in the initiation and progression of UC. On the basis of this, a biomimetic pH-responsive metal organic framework (MOF) carrier was constructed to deliver carbon nanodot-SOD nanozyme and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (CRISPR/Cas9) system for site-specific treatment of UC. In this system, carbon nanodots (C-dots) and CD98 CRISPR/Cas9 plasmid were successfully encapsulated into MOF carrier (ZIF-8 nanoparticles) by a one-pot approach (formed as CCZ), and then camouflaged with macrophage membrane (formed as CCZM). It was worth noting that the C-dot nanozyme showed excellent superoxide dismutase (SOD) enzymatic activity, which could scavenge ROS effectively. As expected, this biomimetic system exhibited pH-responsive, immune escape, and inflammation targeting capability simultaneously. In vitro experiments showed that ROS was significantly eliminated, and CD98 was downregulated by CCZM. In the dextran sulfate sodium salt (DSS)-induced UC model, administration of CCZM significantly ameliorated the inflammation symptoms of mice, including the colon length and pathological parameters such as epithelium integrity and inflammation infiltration. In addition, both in vitro and in vivo results demonstrated that biomimetic nanoparticles effectively reduced the expression of pro-inflammatory cytokines. Overall, this study would provide a promising approach for the precise treatment of UC.
中文翻译:

仿生 MOF 纳米颗粒递送 C-Dot 纳米酶和 CRISPR/Cas9 系统用于溃疡性结肠炎的位点特异性治疗
溃疡性结肠炎 (UC) 是一种病因不明的慢性炎症性肠病 (IBD),影响结肠和直肠。以前的研究发现,活性氧(ROS)过度产生和跨膜糖蛋白 CD98(由SLC3A2编码)) 上调在 UC 的发生和发展中起重要作用。在此基础上,构建了一种仿生pH响应金属有机框架(MOF)载体,用于传递碳纳米点-SOD纳米酶和成簇的规则间隔短回文重复序列(CRISPR)/CRISPR相关蛋白9(Cas9)(CRISPR/Cas9 ) 用于 UC 部位特异性治疗的系统。在该系统中,碳纳米点(C-dots)和 CD98 CRISPR/Cas9 质粒通过一锅法成功封装到 MOF 载体(ZIF-8 纳米颗粒)中(形成为 CCZ),然后用巨噬细胞膜伪装(形成为CCZM)。值得注意的是,C-dot纳米酶表现出优异的超氧化物歧化酶(SOD)酶活性,可有效清除活性氧。正如预期的那样,这种仿生系统表现出对 pH 值敏感、免疫逃逸、和炎症靶向能力同时进行。体外实验表明,ROS被显着消除,CD98被CCZM下调。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。
更新日期:2022-02-09
中文翻译:

仿生 MOF 纳米颗粒递送 C-Dot 纳米酶和 CRISPR/Cas9 系统用于溃疡性结肠炎的位点特异性治疗
溃疡性结肠炎 (UC) 是一种病因不明的慢性炎症性肠病 (IBD),影响结肠和直肠。以前的研究发现,活性氧(ROS)过度产生和跨膜糖蛋白 CD98(由SLC3A2编码)) 上调在 UC 的发生和发展中起重要作用。在此基础上,构建了一种仿生pH响应金属有机框架(MOF)载体,用于传递碳纳米点-SOD纳米酶和成簇的规则间隔短回文重复序列(CRISPR)/CRISPR相关蛋白9(Cas9)(CRISPR/Cas9 ) 用于 UC 部位特异性治疗的系统。在该系统中,碳纳米点(C-dots)和 CD98 CRISPR/Cas9 质粒通过一锅法成功封装到 MOF 载体(ZIF-8 纳米颗粒)中(形成为 CCZ),然后用巨噬细胞膜伪装(形成为CCZM)。值得注意的是,C-dot纳米酶表现出优异的超氧化物歧化酶(SOD)酶活性,可有效清除活性氧。正如预期的那样,这种仿生系统表现出对 pH 值敏感、免疫逃逸、和炎症靶向能力同时进行。体外实验表明,ROS被显着消除,CD98被CCZM下调。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。在葡聚糖硫酸钠盐 (DSS) 诱导的 UC 模型中,CCZM 的给药显着改善了小鼠的炎症症状,包括结肠长度和病理参数,如上皮完整性和炎症浸润。此外,体外和体内结果表明,仿生纳米颗粒有效降低了促炎细胞因子的表达。总体而言,这项研究将为UC的精确治疗提供一种有前途的方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号