当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetate Facilitated Nickel Catalyzed Coupling of Aryl Chlorides and Alkyl Thiols
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-31 , DOI: 10.1021/acscatal.1c04895
Regina M. Oechsner 1 , J. Philipp Wagner 1 , Ivana Fleischer 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-31 , DOI: 10.1021/acscatal.1c04895
Regina M. Oechsner 1 , J. Philipp Wagner 1 , Ivana Fleischer 1
Affiliation
|
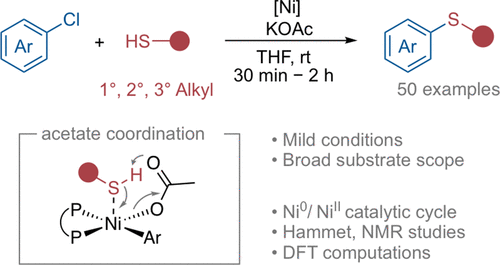
We report a mild, fast, and convenient catalytic system for the coupling of aryl chlorides with primary, secondary, as well as previously challenging tertiary alkyl thiols using an air-stable nickel(II) precatayst in combination with the low-cost base potassium acetate at room temperature. This catalytic system tolerates a variety of functional groups and enables the generation of thioethers for a wide range of substrates, including pharmaceutical compounds in excellent yields. Chemoselective functionalization of disubstituted substrates was demonstrated. Kinetic and NMR studies, as well as DFT computations support a Ni(0)/Ni(II) catalytic cycle and identify the oxidative addition product as the resting state. Acetate coordination and subsequent acetate facilitated formation of a thiolate complex via internal deprotonation play a key role in the catalytic cycle.
中文翻译:

乙酸盐促进镍催化芳基氯和烷基硫醇的偶联
我们报告了一种温和、快速、方便的催化系统,用于芳基氯化物与伯、仲以及以前具有挑战性的叔烷基硫醇的偶联,该系统使用空气稳定的镍 (II) 预催化剂与低成本碱醋酸钾相结合在室温下。这种催化系统可耐受多种官能团,并能够以优异的收率为各种底物(包括药物化合物)生成硫醚。证明了二取代底物的化学选择性功能化。动力学和 NMR 研究以及 DFT 计算支持 Ni(0)/Ni(II) 催化循环,并将氧化加成产物确定为静止状态。
更新日期:2022-01-31
中文翻译:

乙酸盐促进镍催化芳基氯和烷基硫醇的偶联
我们报告了一种温和、快速、方便的催化系统,用于芳基氯化物与伯、仲以及以前具有挑战性的叔烷基硫醇的偶联,该系统使用空气稳定的镍 (II) 预催化剂与低成本碱醋酸钾相结合在室温下。这种催化系统可耐受多种官能团,并能够以优异的收率为各种底物(包括药物化合物)生成硫醚。证明了二取代底物的化学选择性功能化。动力学和 NMR 研究以及 DFT 计算支持 Ni(0)/Ni(II) 催化循环,并将氧化加成产物确定为静止状态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号