Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-01-31 , DOI: 10.1016/j.cej.2022.134946
Serena Poto 1 , Damian Vico van Berkel 1 , Fausto Gallucci 1, 2 , M. Fernanda Neira d'Angelo 1
|
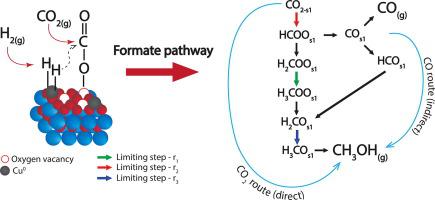
This work addresses the kinetics of the CO2 hydrogenation to methanol over a Cu/CeO2/ZrO2 catalyst studied using single-site, dual-sites and three adsorption sites kinetic models. Physicochemical constraints and statistical indicators are used as tool for model discrimination. The best performing model is used to elucidate the reaction mechanism and the relative roles of the Cu-sites and oxygen vacancies. The results show that the dissociative adsorption of H2 occurs on the Cu0 sites, while CO2 is attracted to the oxygen vacancies created by the CeO2-ZrO2 solid solution. Then, the adsorbed H interacts preferentially with the carbon atom, favouring the so-called “formate” route. The CO formed via the r-WGS reaction could either desorb to the gas phase or react via hydrogenation to methanol. Analysis of the relative contributions of the CO2 and CO hydrogenation (i.e. direct and indirect pathways, respectively) to the methanol synthesis reveals that the latter is in fact preferential at high temperatures (i.e. about 100% of methanol is produced from CO at 260 ⁰C and 30 bar), and it shows an optimum vs the H2:CO2 ratio (c.a. 7 at 200 ⁰C and 30 bar), which corresponds to the saturation of the Cu0 sites with H2. Thus, this work provides an essential tool (i.e., kinetic model) for the design of reactors and processes based on novel catalysts, and importantly, it offers a deeper understanding of the reaction mechanism as basis for further catalyst development.
中文翻译:

在 CuO/CeO2/ZrO2 催化剂上由 CO2 和 H2 合成甲醇的动力学模型:CO2 和 CO 加氢的作用
这项工作解决了使用单中心、双中心和三吸附中心动力学模型研究的 Cu/CeO 2 /ZrO 2催化剂上 CO 2加氢制甲醇的动力学。物理化学约束和统计指标被用作模型识别的工具。最佳性能模型用于阐明反应机理以及 Cu 位点和氧空位的相对作用。结果表明,H 2的解离吸附发生在Cu 0位点,而CO 2被CeO 2 -ZrO 2产生的氧空位吸引。实在的方法。然后,吸附的 H 优先与碳原子相互作用,有利于所谓的“甲酸盐”路线。通过 r-WGS 反应形成的 CO 可以解吸到气相中,也可以通过加氢反应生成甲醇。分析 CO 2和 CO 加氢(即分别为直接和间接途径)对甲醇合成的相对贡献表明,后者实际上在高温下优先(即约 100% 的甲醇在 260 ⁰C 下由 CO 生产)和 30 bar),它显示了与 H 2 :CO 2比(ca 7 at 200 ⁰C 和 30 bar)的最佳值,这对应于 Cu 0位点与 H 2的饱和度. 因此,这项工作为基于新型催化剂的反应器和工艺设计提供了必不可少的工具(即动力学模型),更重要的是,它提供了对反应机理的更深入理解,作为进一步催化剂开发的基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号