当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective electrophilic di- and monofluorinations for the synthesis of 4-difluoromethyl and 4-fluoromethyl quinazolin(thi)ones by a Selectfluor-triggered multi-component reaction
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-01-20 , DOI: 10.1039/d1qo01728d Ruiqin Zhang 1, 2 , Renchao Ma 1 , Qinjiao Fu 1 , Rener Chen 1 , Zhiming Wang 1 , Lei Wang 1 , Yongmin Ma 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-01-20 , DOI: 10.1039/d1qo01728d Ruiqin Zhang 1, 2 , Renchao Ma 1 , Qinjiao Fu 1 , Rener Chen 1 , Zhiming Wang 1 , Lei Wang 1 , Yongmin Ma 1, 2
Affiliation
|
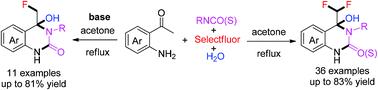
A simple and efficient domino protocol for the selective synthesis of 4-difluoromethyl and 4-fluoromethyl quinazolin(thi)ones was established from readily available 2-aminoacetophenones and iso(thio)cyanates mediated by Selectfluor. The reaction outcomes are restricted by the reaction environment. Without the use of a base, gem-difluoro-oxylated quinazolin(thi)ones were afforded effectively as a sole product. In contrast, only monofluoro-oxylated analogues were obtained under basic conditions.
中文翻译:

选择性亲电二氟化和单氟化,用于通过 Selectfluor 触发的多组分反应合成 4-二氟甲基和 4-氟甲基喹唑啉(噻)酮
一种简单有效的多米诺骨牌协议,用于选择性合成 4-二氟甲基和 4-氟甲基喹唑啉(噻)酮,由 Selectfluor 介导的现成的 2-氨基苯乙酮和异(硫)氰酸酯建立。反应结果受到反应环境的限制。在不使用碱的情况下,可以有效地提供偕二氟氧化喹唑啉(噻)酮作为唯一产品。相反,在碱性条件下仅获得单氟氧化类似物。
更新日期:2022-01-20
中文翻译:

选择性亲电二氟化和单氟化,用于通过 Selectfluor 触发的多组分反应合成 4-二氟甲基和 4-氟甲基喹唑啉(噻)酮
一种简单有效的多米诺骨牌协议,用于选择性合成 4-二氟甲基和 4-氟甲基喹唑啉(噻)酮,由 Selectfluor 介导的现成的 2-氨基苯乙酮和异(硫)氰酸酯建立。反应结果受到反应环境的限制。在不使用碱的情况下,可以有效地提供偕二氟氧化喹唑啉(噻)酮作为唯一产品。相反,在碱性条件下仅获得单氟氧化类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号