当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Hydrogenation toward Chiral 3-Aryloxy Tetrahydrofurans Enabled by Spiro Ir-PNN Catalysts Containing an Unusual 5-Substituted Chiral Oxazoline Unit
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-28 , DOI: 10.1021/acscatal.1c05746
Xue-Song Gu 1 , Ying Xiong 1 , Fan Yang 1 , Na Yu 1 , Pu-Cha Yan 2 , Jian-Hua Xie 1 , Qi-Lin Zhou 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-28 , DOI: 10.1021/acscatal.1c05746
Xue-Song Gu 1 , Ying Xiong 1 , Fan Yang 1 , Na Yu 1 , Pu-Cha Yan 2 , Jian-Hua Xie 1 , Qi-Lin Zhou 1
Affiliation
|
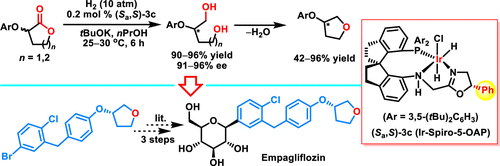
An iridium-catalyzed asymmetric hydrogenation of racemic α-aryloxy lactones involving dynamic kinetic resolution was successfully implemented by chiral spiro PNN-ligands containing a C5-substituted chiral oxazoline unit. This protocol along with simple dehydration constitutes the most straightforward approach to prepare enantiomerically enriched 3-aryloxy tetrahydrofurans, which are prevalent structural motifs in many approved drugs and clinical candidates. The hydrogenation step features high yields (90–96%), low catalyst loading (0.2–0.1 mol %), and high enantioselectivities (91–96% ee), and the dehydration step is absolutely stereospecific with high yields (up to 96%) under the mild condition. Moreover, the synthetic utility was further proved by the formal enantioselective synthesis of empagliflozin and production of 3-aryloxy pyrrolidine units.
中文翻译:

含有不寻常的 5-取代手性恶唑啉单元的 Spiro Ir-PNN 催化剂实现对手性 3-芳氧基四氢呋喃的对映选择性氢化
通过含有 C5 取代的手性恶唑啉单元的手性螺环 PNN-配体成功实现了涉及动态动力学拆分的外消旋 α-芳氧基内酯的铱催化不对称氢化。该协议与简单的脱水一起构成了制备对映体富集的 3-芳氧基四氢呋喃的最直接方法,这是许多已批准药物和临床候选药物中普遍存在的结构基序。氢化步骤具有高产率 (90–96%)、低催化剂负载 (0.2–0.1 mol%) 和高对映选择性 (91–96% ee) 的特点,脱水步骤是绝对立体有择的,产率高(高达 96% ) 在温和的条件下。而且,
更新日期:2022-01-28
中文翻译:

含有不寻常的 5-取代手性恶唑啉单元的 Spiro Ir-PNN 催化剂实现对手性 3-芳氧基四氢呋喃的对映选择性氢化
通过含有 C5 取代的手性恶唑啉单元的手性螺环 PNN-配体成功实现了涉及动态动力学拆分的外消旋 α-芳氧基内酯的铱催化不对称氢化。该协议与简单的脱水一起构成了制备对映体富集的 3-芳氧基四氢呋喃的最直接方法,这是许多已批准药物和临床候选药物中普遍存在的结构基序。氢化步骤具有高产率 (90–96%)、低催化剂负载 (0.2–0.1 mol%) 和高对映选择性 (91–96% ee) 的特点,脱水步骤是绝对立体有择的,产率高(高达 96% ) 在温和的条件下。而且,

































 京公网安备 11010802027423号
京公网安备 11010802027423号