Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing the Chemical Reaction Properties of a SiHCl3 Pyrolysis System by the ReaxFF Molecular Dynamics Method
ACS Omega ( IF 3.7 ) Pub Date : 2022-01-28 , DOI: 10.1021/acsomega.1c03998 Yanping Li 1, 2 , Dazhou Yan 1, 2, 3 , Tao Yang 1, 2 , Guosheng Wen 1, 2 , Xin Yao 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2022-01-28 , DOI: 10.1021/acsomega.1c03998 Yanping Li 1, 2 , Dazhou Yan 1, 2, 3 , Tao Yang 1, 2 , Guosheng Wen 1, 2 , Xin Yao 1, 2
Affiliation
|
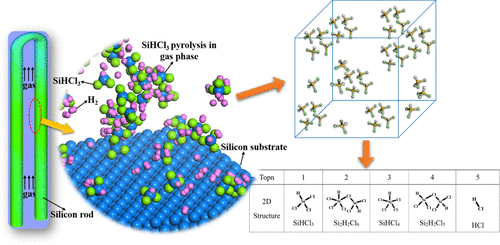
The pyrolysis kinetics of SiHCl3 and its reaction mechanism are essential for the chemical vapor deposition process in polysilicon industries. However, due to the high temperature and lack of in situ experimental detection technology, it is difficult to carry out experimental research on the pyrolysis kinetics of SiHCl3. In this work, reactive force field molecular dynamics simulations of SiHCl3 pyrolysis were performed to investigate the effect of temperature on the pyrolysis kinetics of SiHCl3 at the atomistic scale in a wide temperature range (1000–2000 K). The lumped Si clusters containing more than five Si atoms tended to appear at the later period of the reaction under a temperature lower than 1300 K, some of which even possessed polycyclic structures; nevertheless, small ones with less than two Si atoms such as SiHCl2 and HCl tended to emerge under a high temperature. The changes of partial energy terms with time evolution under various temperatures were proved to be rooted in the distribution of intermediates based on the momentary simulation period. In general, the reaction network at a low temperature was more complicated than that at a high temperature, resulting from the fact that more chemical events and intermediates came into existence, and the maximum number of Si atoms in one single molecule/radical was observed under a low temperature than that under a high temperature. As to the variation of SiHCl3 with the progress of the reaction, the linear fitting tendency disappeared under the temperature above 1300 K, which changed in fluctuation with the further elevation of temperature, elucidating the fact that SiHCl3 can act as a product and not just as a reactant to participate in elementary chemical events frequently.
中文翻译:

用 ReaxFF 分子动力学方法揭示 SiHCl3 热解系统的化学反应性质
SiHCl 3的热解动力学及其反应机理对于多晶硅工业中的化学气相沉积过程至关重要。但由于高温和缺乏原位实验检测技术,难以开展SiHCl 3热解动力学的实验研究。在这项工作中,对 SiHCl 3热解的反应力场分子动力学进行了模拟,以研究温度对 SiHCl 3热解动力学的影响。在很宽的温度范围(1000-2000 K)的原子尺度上。在低于 1300 K 的温度下,含有 5 个以上 Si 原子的集总 Si 簇往往出现在反应后期,其中一些甚至具有多环结构;然而,具有少于两个 Si 原子的小分子,例如 SiHCl 2和 HCl 往往在高温下出现。基于瞬时模拟周期,证明了不同温度下部分能量项随时间演化的变化源于中间体的分布。一般来说,低温下的反应网络比高温下的反应网络更复杂,这是由于存在更多的化学事件和中间体,并且在单个分子/自由基中观察到的最大硅原子数是在低温下比高温下。SiHCl 3随着反应进行的变化,在1300 K以上的温度下线性拟合趋势消失,随着温度的进一步升高而波动变化,说明了SiHCl3可以作为产物而不仅仅是作为反应物频繁参与基本的化学事件。
更新日期:2022-02-08
中文翻译:

用 ReaxFF 分子动力学方法揭示 SiHCl3 热解系统的化学反应性质
SiHCl 3的热解动力学及其反应机理对于多晶硅工业中的化学气相沉积过程至关重要。但由于高温和缺乏原位实验检测技术,难以开展SiHCl 3热解动力学的实验研究。在这项工作中,对 SiHCl 3热解的反应力场分子动力学进行了模拟,以研究温度对 SiHCl 3热解动力学的影响。在很宽的温度范围(1000-2000 K)的原子尺度上。在低于 1300 K 的温度下,含有 5 个以上 Si 原子的集总 Si 簇往往出现在反应后期,其中一些甚至具有多环结构;然而,具有少于两个 Si 原子的小分子,例如 SiHCl 2和 HCl 往往在高温下出现。基于瞬时模拟周期,证明了不同温度下部分能量项随时间演化的变化源于中间体的分布。一般来说,低温下的反应网络比高温下的反应网络更复杂,这是由于存在更多的化学事件和中间体,并且在单个分子/自由基中观察到的最大硅原子数是在低温下比高温下。SiHCl 3随着反应进行的变化,在1300 K以上的温度下线性拟合趋势消失,随着温度的进一步升高而波动变化,说明了SiHCl3可以作为产物而不仅仅是作为反应物频繁参与基本的化学事件。































 京公网安备 11010802027423号
京公网安备 11010802027423号