当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Optically Active 3-Cyclohexene-1-carboxylic Acid Utilizing Lactic Ester as a Chiral Auxiliary in the Diastereoselective Diels–Alder Reaction
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-28 , DOI: 10.1021/acs.oprd.1c00430 Hidenori Ochiai 1 , Wakana Hayashi 1 , Akira Nishiyama 1 , Ryunosuke Fujita 1 , Shunichi Kubota 1 , Miwa Sasagawa 1 , Tatsuya Nishi 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-28 , DOI: 10.1021/acs.oprd.1c00430 Hidenori Ochiai 1 , Wakana Hayashi 1 , Akira Nishiyama 1 , Ryunosuke Fujita 1 , Shunichi Kubota 1 , Miwa Sasagawa 1 , Tatsuya Nishi 1
Affiliation
|
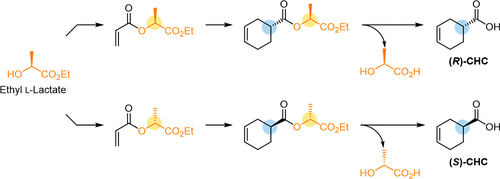
The optically active 3-cyclohexene-1-carboxylic acid was synthesized through a TiCl4-catalyzed diastereoselective Diels–Alder reaction utilizing lactic acid ester as a chiral auxiliary, which can be removed by washing with H2O. The (S)- and (R)-isomers were both derived from easily available ethyl l-lactate.
中文翻译:

在非对映选择性 Diels-Alder 反应中以乳酸为手性助剂不对称合成光学活性 3-Cyclohexene-1-羧酸
使用乳酸酯作为手性助剂,通过 TiCl 4催化的非对映选择性 Diels-Alder 反应合成光学活性的 3-环己烯-1-羧酸,可以通过 H 2 O洗涤去除。 ( R )-异构体均衍生自容易获得的L-乳酸乙酯。
更新日期:2022-01-28
中文翻译:

在非对映选择性 Diels-Alder 反应中以乳酸为手性助剂不对称合成光学活性 3-Cyclohexene-1-羧酸
使用乳酸酯作为手性助剂,通过 TiCl 4催化的非对映选择性 Diels-Alder 反应合成光学活性的 3-环己烯-1-羧酸,可以通过 H 2 O洗涤去除。 ( R )-异构体均衍生自容易获得的L-乳酸乙酯。






































 京公网安备 11010802027423号
京公网安备 11010802027423号