当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure identification and analysis of the suspected chemical precursor of 2-fluorodeschloroketamine and its decomposition products
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2022-01-27 , DOI: 10.1002/dta.3229 Xuan Luo 1, 2 , Di Zhang 1 , Qiulian Luo 1 , Kejian Huang 3 , Xiaofeng Liu 3 , Ning Yang 3 , Zuzeng Qin 1 , Chunli Feng 1 , Junbo Li 4
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2022-01-27 , DOI: 10.1002/dta.3229 Xuan Luo 1, 2 , Di Zhang 1 , Qiulian Luo 1 , Kejian Huang 3 , Xiaofeng Liu 3 , Ning Yang 3 , Zuzeng Qin 1 , Chunli Feng 1 , Junbo Li 4
Affiliation
|
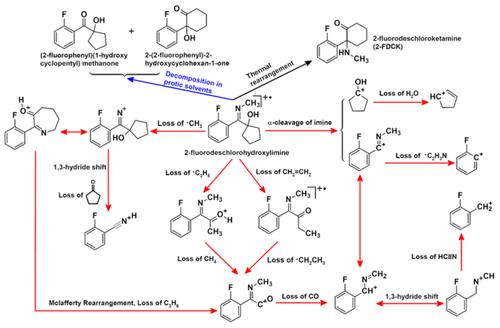
In this work, 1-[(2″-fluorophenyl)(methylimino)methyl]cyclopentan-1-ol (2-fluorodeschlorohydroxylimine) was identified as a suspected chemical precursor of 2-fluorodeschloroketamine (2-FDCK) using gas chromatography–mass spectrometry (GC–MS) and gas chromatography-quadrupole/time-of-flight mass spectrometry (GC-Q/TOF-MS) and comparing the data with those of ketamine and its chemical precursor, hydroxylimine. Furthermore, the entire fragmentation pathway of 2-fluorodeschlorohydroxylimine was theorized from the GC–MS spectrum recorded using an electron ionization (EI) source, and the mechanisms and decomposition pathways of 2-fluorodeschlorohydroxylimine were elucidated. In protic solvents, the nitrogen atom in the C═N group of 2-fluorodeschlorohydroxylimine underwent a protonation reaction. Thereafter, the traces of water present in protic solvents promoted the hydrolysis of the protonated imine, and a carbon cation was obtained following the loss of methylamine. The carbon cation could follow the classical decomposition mechanism of imines and yield an α-hydroxyl ketone, which was the major decomposition product, (2′-fluorophenyl)(1″-hydroxycyclopentyl)methanone. The cation could also undergo a loop expansion rearrangement and yield another α-hydroxyl ketone, 2-(2′-fluorophenyl)-2-hydroxycyclohexan-1-one. The structures of the two aforementioned decomposition products were elucidated using several techniques including theoretical calculation, GC–MS, nuclear magnetic resonance (NMR), the prediction and assistance elucidation functions of ACDLabs-Structure Elucidator Suite, and the virtual separation technology of diffusion-ordered spectroscopy. The aforementioned study revealed important information about the chemical precursor of 2-FDCK and its decomposition. Furthermore, a set of methods for the qualitative analysis of 2-fluorodeschlorohydroxylimine were established, which facilitated accurate analysis of 2-fluorodeschlorohydroxylimine samples following decomposition or destruction.
中文翻译:

2-氟去氯氯胺酮及其分解产物疑似化学前体的结构鉴定与分析
在这项工作中,使用气相色谱-质谱法将 1-[(2″-fluorophenyl)(methylimino)methyl]cyclopentan-1-ol (2-fluorodeschlorohydroxylimine) 鉴定为 2-fluorodeschloroketamine (2-FDCK) 的可疑化学前体(GC-MS)和气相色谱-四极杆/飞行时间质谱(GC-Q/TOF-MS),并将数据与氯胺酮及其化学前体羟亚胺的数据进行比较。此外,从使用电子电离 (EI) 源记录的 GC-MS 谱图推导了 2-氟脱氯羟亚胺的整个裂解途径,阐明了 2-氟脱氯羟亚胺的分解机理和分解途径。在质子溶剂中,2-氟脱氯羟亚胺的 C=N 基团中的氮原子发生质子化反应。此后,质子溶剂中存在的痕量水促进了质子化亚胺的水解,并且在失去甲胺后获得了碳阳离子。碳阳离子可以遵循亚胺的经典分解机理并产生α-羟基酮,这是主要的分解产物,(2'-氟苯基)(1''-羟基环戊基)甲酮。该阳离子还可以进行环扩展重排并产生另一种α-羟基酮,2-(2'-氟苯基)-2-羟基环己-1-酮。使用理论计算、GC-MS、核磁共振(NMR)、ACDLabs-Structure Elucidator Suite的预测和辅助解析功能、扩散有序虚拟分离技术等多种技术对上述两种分解产物的结构进行了解析。光谱学。上述研究揭示了有关 2-FDCK 化学前体及其分解的重要信息。此外,还建立了一套2-氟脱氯羟亚胺定性分析方法,有助于对分解或破坏后的2-氟脱氯羟亚胺样品进行准确分析。
更新日期:2022-01-27
中文翻译:

2-氟去氯氯胺酮及其分解产物疑似化学前体的结构鉴定与分析
在这项工作中,使用气相色谱-质谱法将 1-[(2″-fluorophenyl)(methylimino)methyl]cyclopentan-1-ol (2-fluorodeschlorohydroxylimine) 鉴定为 2-fluorodeschloroketamine (2-FDCK) 的可疑化学前体(GC-MS)和气相色谱-四极杆/飞行时间质谱(GC-Q/TOF-MS),并将数据与氯胺酮及其化学前体羟亚胺的数据进行比较。此外,从使用电子电离 (EI) 源记录的 GC-MS 谱图推导了 2-氟脱氯羟亚胺的整个裂解途径,阐明了 2-氟脱氯羟亚胺的分解机理和分解途径。在质子溶剂中,2-氟脱氯羟亚胺的 C=N 基团中的氮原子发生质子化反应。此后,质子溶剂中存在的痕量水促进了质子化亚胺的水解,并且在失去甲胺后获得了碳阳离子。碳阳离子可以遵循亚胺的经典分解机理并产生α-羟基酮,这是主要的分解产物,(2'-氟苯基)(1''-羟基环戊基)甲酮。该阳离子还可以进行环扩展重排并产生另一种α-羟基酮,2-(2'-氟苯基)-2-羟基环己-1-酮。使用理论计算、GC-MS、核磁共振(NMR)、ACDLabs-Structure Elucidator Suite的预测和辅助解析功能、扩散有序虚拟分离技术等多种技术对上述两种分解产物的结构进行了解析。光谱学。上述研究揭示了有关 2-FDCK 化学前体及其分解的重要信息。此外,还建立了一套2-氟脱氯羟亚胺定性分析方法,有助于对分解或破坏后的2-氟脱氯羟亚胺样品进行准确分析。

































 京公网安备 11010802027423号
京公网安备 11010802027423号