当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactive Vapor-Phase Additives toward Destabilizing γ-Mg(BH4)2 for Improved Hydrogen Release
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-01-27 , DOI: 10.1021/acsaem.1c03128
Nicholas A. Strange 1, 2 , Noemi Leick 1 , Sarah Shulda 1 , Andreas Schneemann 3 , Vitalie Stavila 3 , Andrew S. Lipton 4 , Michael F. Toney 2, 5 , Thomas Gennett 1, 6 , Steven T. Christensen 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-01-27 , DOI: 10.1021/acsaem.1c03128
Nicholas A. Strange 1, 2 , Noemi Leick 1 , Sarah Shulda 1 , Andreas Schneemann 3 , Vitalie Stavila 3 , Andrew S. Lipton 4 , Michael F. Toney 2, 5 , Thomas Gennett 1, 6 , Steven T. Christensen 1
Affiliation
|
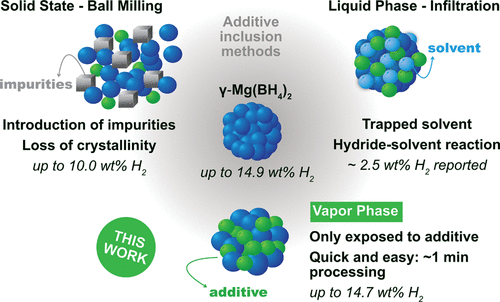
Magnesium borohydride (Mg(BH4)2) is a promising candidate for material-based hydrogen storage due to its high hydrogen gravimetric/volumetric capacities and potential for dehydrogenation reversibility. Currently, slow dehydrogenation kinetics and the formation of intermediate polyboranes deter its application in clean energy technologies. In this study, a novel approach for modifying the physicochemical properties of Mg(BH4)2 is described, which involves the addition of reactive molecules in the vapor phase. This process enables the investigation of a new class of additive molecules for material-based hydrogen storage. The effects of four molecules (BBr3, Al2(CH3)6, TiCl4, and N2H4) with varying degrees of electrophilicity are examined to infer how the chemical reactivity can be used to tune the additive–Mg(BH4)2 interaction and optimize the release of hydrogen at lower temperatures. Control over the amounts of additive exposure to Mg(BH4)2 is shown to prevent degradation of the bulk γ-Mg(BH4)2 crystal structure and loss of hydrogen capacity. Trimethylaluminum provides the most encouraging results on Mg(BH4)2, maintaining 97% of the starting theoretical Mg(BH4)2 hydrogen content and demonstrating hydrogen release at 115 °C. These results firmly establish the efficacy of this approach toward controlling the properties of Mg(BH4)2 and provide a new path forward for additive-based modification of hydrogen storage materials.
中文翻译:

用于使 γ-Mg(BH4)2 不稳定以改善氢气释放的反应性气相添加剂
硼氢化镁 (Mg(BH 4 ) 2 ) 因其高的氢重量/体积容量和脱氢可逆性的潜力而成为基于材料的储氢的有希望的候选者。目前,缓慢的脱氢动力学和中间聚硼烷的形成阻碍了其在清洁能源技术中的应用。在这项研究中,描述了一种改变 Mg(BH 4 ) 2物理化学性质的新方法,其中包括在气相中添加反应性分子。该过程能够研究一类用于基于材料的储氢的新型添加剂分子。四种分子(BBr 3、Al 2 (CH 3) 6、TiCl 4和N 2 H 4 ) 具有不同程度的亲电性,以推断化学反应性如何用于调节添加剂-Mg(BH 4 ) 2相互作用并优化低温下氢的释放。控制暴露于 Mg(BH 4 ) 2的添加剂的量显示可防止本体 γ-Mg(BH 4 ) 2晶体结构的退化和氢容量的损失。三甲基铝在 Mg(BH 4 ) 2上提供了最令人鼓舞的结果,保持了起始理论 Mg(BH 4 )的 97%2氢含量并证明在 115 °C 时有氢释放。这些结果牢固地确立了这种方法对控制 Mg(BH 4 ) 2性能的有效性,并为基于添加剂的储氢材料改性提供了新的途径。
更新日期:2022-01-27
中文翻译:

用于使 γ-Mg(BH4)2 不稳定以改善氢气释放的反应性气相添加剂
硼氢化镁 (Mg(BH 4 ) 2 ) 因其高的氢重量/体积容量和脱氢可逆性的潜力而成为基于材料的储氢的有希望的候选者。目前,缓慢的脱氢动力学和中间聚硼烷的形成阻碍了其在清洁能源技术中的应用。在这项研究中,描述了一种改变 Mg(BH 4 ) 2物理化学性质的新方法,其中包括在气相中添加反应性分子。该过程能够研究一类用于基于材料的储氢的新型添加剂分子。四种分子(BBr 3、Al 2 (CH 3) 6、TiCl 4和N 2 H 4 ) 具有不同程度的亲电性,以推断化学反应性如何用于调节添加剂-Mg(BH 4 ) 2相互作用并优化低温下氢的释放。控制暴露于 Mg(BH 4 ) 2的添加剂的量显示可防止本体 γ-Mg(BH 4 ) 2晶体结构的退化和氢容量的损失。三甲基铝在 Mg(BH 4 ) 2上提供了最令人鼓舞的结果,保持了起始理论 Mg(BH 4 )的 97%2氢含量并证明在 115 °C 时有氢释放。这些结果牢固地确立了这种方法对控制 Mg(BH 4 ) 2性能的有效性,并为基于添加剂的储氢材料改性提供了新的途径。



































 京公网安备 11010802027423号
京公网安备 11010802027423号