当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Straightforward synthesis of thiazolo[5,4-c]isoquinolines from dithiooxamide and 2-halobenzaldehydes
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-01-20 , DOI: 10.1039/d1nj05536d Letícia D. Costa 1 , Samuel Guieu 1, 2 , Maria do Amparo F. Faustino 1 , Augusto C. Tomé 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-01-20 , DOI: 10.1039/d1nj05536d Letícia D. Costa 1 , Samuel Guieu 1, 2 , Maria do Amparo F. Faustino 1 , Augusto C. Tomé 1
Affiliation
|
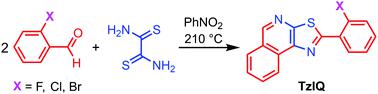
The reaction of dithiooxamide with aromatic aldehydes is a well-known method for the preparation of thiazolo[5,4-d]thiazoles (TzTz). However, we report here that, using adequately substituted 2-halobenzaldehydes, this reaction may afford selectively thiazolo[5,4-c]isoquinolines (TzIQ) or mixtures of TzTz and TzIQ. The use of lanthanum(III) triflate as the catalyst favors the formation of TzIQ. The results obtained using a large number of substituted benzaldehydes showed that the nature, number and position of halogen atoms and other substituents on the aldehyde have a great impact on the reaction outcome. The structures of six TzTz and six TzIQ were unveiled by single-crystal X-ray diffraction.
中文翻译:

由二硫代草酰胺和 2-卤代苯甲醛直接合成噻唑并[5,4-c]异喹啉
二硫代草酰胺与芳香醛的反应是制备噻唑并[5,4- d ]噻唑(TzTz)的众所周知的方法。然而,我们在此报告,使用充分取代的 2-卤代苯甲醛,该反应可以选择性地提供噻唑并[5,4- c ] 异喹啉 (TzIQ) 或 TzTz 和 TzIQ 的混合物。使用三氟甲磺酸镧( III )作为催化剂有利于TzIQ的形成。使用大量取代苯甲醛得到的结果表明,醛上卤素原子和其他取代基的性质、数量和位置对反应结果有很大影响。通过单晶X射线衍射揭示了6个TzTz和6个TzIQ的结构。
更新日期:2022-01-20
中文翻译:

由二硫代草酰胺和 2-卤代苯甲醛直接合成噻唑并[5,4-c]异喹啉
二硫代草酰胺与芳香醛的反应是制备噻唑并[5,4- d ]噻唑(TzTz)的众所周知的方法。然而,我们在此报告,使用充分取代的 2-卤代苯甲醛,该反应可以选择性地提供噻唑并[5,4- c ] 异喹啉 (TzIQ) 或 TzTz 和 TzIQ 的混合物。使用三氟甲磺酸镧( III )作为催化剂有利于TzIQ的形成。使用大量取代苯甲醛得到的结果表明,醛上卤素原子和其他取代基的性质、数量和位置对反应结果有很大影响。通过单晶X射线衍射揭示了6个TzTz和6个TzIQ的结构。































 京公网安备 11010802027423号
京公网安备 11010802027423号