Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A General Way to Spiro-Annulated 2-Benzoxepines via Rh2(esp)2-Catalyzed [5+2] Cycloaddition of Diazo Arylidene Succinimides to Ketones
Synthesis ( IF 2.2 ) Pub Date : 2022-01-26 , DOI: 10.1055/s-0037-1610790 Mikhail Krasavin 1, 2 , Dmitry Dar’in 1 , Anastasia Vepreva 1 , Grigory Kantin 1
中文翻译:

Rh2(esp)2 催化重氮亚芳基琥珀酰亚胺与酮的 [5+2] 环加成螺环化 2-苯氧西平的一般方法
更新日期:2022-01-27
Synthesis ( IF 2.2 ) Pub Date : 2022-01-26 , DOI: 10.1055/s-0037-1610790 Mikhail Krasavin 1, 2 , Dmitry Dar’in 1 , Anastasia Vepreva 1 , Grigory Kantin 1
Affiliation
|
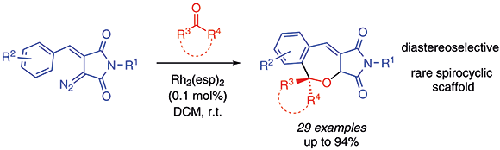
The formation of spirocyclic 2-benzoxepines by Rh2(esp)2-catalyzed decomposition of diazo arylidene succinimides in the presence of ketones was investigated. This transformation, which is a formal [5+2] cycloaddition of styryl rhodium carbenes to the carbonyl group, occurs in high yields under mild conditions, with high carbonyl substrate tolerance and diastereoselectivity. The developed general method opens access to rare spiro (hetero)cyclic scaffolds with great potential in drug discovery.
中文翻译:

Rh2(esp)2 催化重氮亚芳基琥珀酰亚胺与酮的 [5+2] 环加成螺环化 2-苯氧西平的一般方法
研究了在酮存在下Rh 2 (esp) 2催化重氮亚芳基琥珀酰亚胺分解形成螺环2-苯并氧杂环庚烷。这种转化是苯乙烯基铑卡宾与羰基的正式 [5+2] 环加成,在温和条件下以高产率发生,具有高羰基底物耐受性和非对映选择性。开发的通用方法打开了获得罕见螺(杂)环支架的途径,在药物发现方面具有巨大潜力。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号