Synthesis ( IF 2.2 ) Pub Date : 2022-01-26 , DOI: 10.1055/s-0040-1719873 Rachel L. Howells 1 , Scott G. Lamont 1 , Thomas M. McGuire 1 , Samantha Hughes 1 , Rachel Borrows 1 , Gary Fairley 1 , Lyman J. L. Feron 1 , Ryan D. R. Greenwood 1 , Eva Lenz 1 , Emma Grant 2 , Iain Simpson 2
|
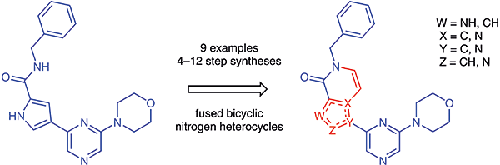
As part of a drug discovery program, 4-pyrazin-2-yl-1H-pyrrole-2-carboxamides were accessed along with a number of bicyclic analogues. Routes to these compounds were largely absent from the scientific literature. The synthesis of a 4-(pyrazin-2-yl)-1H-pyrrole-2-carboxamide and several fused bicyclic analogues all using standard procedures (SNAr, borylation, C–C cross couplings, hydrolysis, amide bond formation, cyclisation, halogenation, and alkylation) from readily available starting materials is reported. The synthetic sequences range from 4–12 steps per final compound, with yields of isolated intermediates ranging from 20 to ∼100%.
中文翻译:

新型吡嗪取代的 1H-吡咯-2-甲酰胺和相关束缚杂环的合成
作为药物发现计划的一部分,4-pyrrazin-2-yl-1 H -pyrrole-2-carboxamides 与许多双环类似物一起被使用。科学文献中基本上没有找到这些化合物的途径。4-(pyrazin-2-yl)-1 H -pyrrole-2-carboxamide 和几种稠合双环类似物的合成均使用标准程序(S N Ar、硼化、C-C 交叉偶联、水解、酰胺键形成、环化,卤化和烷基化)从容易获得的起始材料报道。每个最终化合物的合成序列范围为 4-12 步,分离中间体的产率范围为 20% 至 ∼100%。































 京公网安备 11010802027423号
京公网安备 11010802027423号