当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Decrypting Catalytic NOX Activation and Poison Fragmentation Routes Boosted by Mono- and Bi-Dentate Surface SO32–/SO42– Modifiers under a SO2-Containing Flue Gas Stream
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-25 , DOI: 10.1021/acscatal.1c04611 Jongsik Kim 1 , Dong Ho Kim 1, 2 , Jinseon Park 3 , Keunhong Jeong 3 , Heon Phil Ha 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-01-25 , DOI: 10.1021/acscatal.1c04611 Jongsik Kim 1 , Dong Ho Kim 1, 2 , Jinseon Park 3 , Keunhong Jeong 3 , Heon Phil Ha 1
Affiliation
|
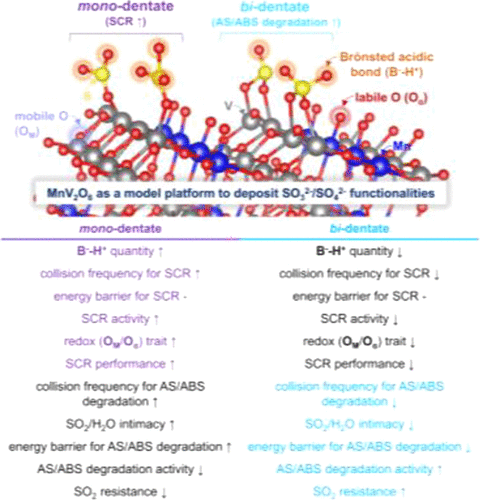
SOA2– (A = 3–4; B–) functionalities are anchored on metal oxides used to catalyze NH3-assisted selective NOX reduction (SCR) for a SO2-bearing feed gas stream. SOA2– species act as conjugate bases of Brönsted acidic bonds (B––H+) and modifiers of redox sites (M(n–1)+–O–), both of which are combined to dictate the activities of SCR (−rNOX) and ammonium (bi) sulfate (AS/ABS) poison degradation (−rAS/ABS) at low temperatures. Nonetheless, their pathways have been barely clarified and underexplored, while questioning catalytic significance of mono-dentate or bi-dentate SOA2– species in dominating −rNOX and −rAS/ABS. While using Sb-promoted MnV2O6 as a reservoir of SOA2– functionalities with distinct binding arrays, elementary stages for the SCR and AS/ABS degradation were proposed, thermodynamically assessed, and analyzed using kinetic control runs in tandem with density functional theory calculations. These allowed for the conclusions that the reaction stage between B––H+•••NH3•••O––M(n–1)+ and gaseous NO and the liberation stage of H2O/SO2 from B–•••H2O•••SO2•••H2O via dissociative desorption are endothermic and dominate −rNOX and −rAS/ABS as the rate-determining steps of the SCR and AS/ABS degradation, respectively. In addition, mono-dentate and bi-dentate SOA2– species are verified central in directing −rNOX and −rAS/ABS by elevating collision frequency between B––H+•••NH3•••O––M(n–1)+ and NO and declining the energy barrier required for dissociative H2O/SO2 desorption for the SCR and AS/ABS degradation, respectively. In particular, mono-dentate SOA2– functionalities can improve the overall redox trait of the surface, thereby substantially promoting its low-temperature SCR performance under a SO2-excluding feed gas stream. Meanwhile, bi-dentate SOA2– functionalities can slightly improve the overall redox trait of the surface, yet, can readily degrade AS/ABS by accelerating the endothermic fragmentation of S2O72– innate to ammonium pyrosulfate, while compensating for the moderate efficiency in fragmenting NH4+ of ammonium pyrosulfate via Eley–Rideal-type SCR. This can significantly elevate the SCR performance of the bi-dentate SOA2–-containing surface under a SO2-including feed gas stream alongside with the promotion of its long-term stability at low temperatures. These can be adaptable and exploited in discovering/amending a host of metal oxides (or vanadates) imperatively functionalized with SOA2– or poisoned with AS/ABS under low thermal energies.
中文翻译:

解密含 SO2 烟道气流下单齿和双齿表面 SO32–/SO42– 改性剂促进的催化 NOX 活化和毒物破碎途径
SO A 2– ( A = 3–4; B – ) 官能团固定在金属氧化物上,该金属氧化物用于催化含 SO 2原料气流的 NH 3辅助选择性 NO X还原 (SCR) 。SO A 2-物质作为布朗斯台德酸键 (B – –H + ) 的共轭碱基和氧化还原位点 (M ( n– 1)+ –O – ) 的修饰剂,两者结合起来决定 SCR 的活性 ( − r NO X ) 和硫酸铵 (双) (AS/ABS) 毒物降解 (− r AS/ABS) 在低温下。尽管如此,它们的途径几乎没有得到澄清和探索,同时质疑单齿或双齿 SO A 2-物种在主导 - r NO X和 - r AS/ABS中的催化意义。在使用 Sb 促进的 MnV 2 O 6作为 SO A 2的储层时-提出了具有不同结合阵列、SCR 和 AS/ABS 降解的基本阶段的功能,并使用动力学控制运行与密度泛函理论计算进行了热力学评估和分析。由此得出以下结论:B – –H + •••NH 3 •••O – –M ( n –1)+与气态 NO 之间的反应阶段和 B – H 2 O /SO 2的释放阶段– •••H 2 O•••SO 2 •••H 2 O 通过解离解吸吸热并占主导地位 - r NO X和 -r AS/ABS分别作为 SCR 和 AS/ABS 退化的速率决定步骤。此外,通过提高 B – –H + •••NH 3 •••O –之间的碰撞频率,验证了单齿和双齿 SO A 2–物质在引导 - r NO X和 - r AS/ABS方面的核心作用–M ( n –1)+和 NO 并分别降低 SCR 和 AS/ABS 降解的解离 H 2 O/SO 2解吸所需的能垒。特别是单声道-齿状SO A 2-官能团可以改善表面的整体氧化还原特性,从而显着提高其在不含SO 2的原料气流下的低温SCR性能。同时,双齿 SO A 2-官能团可以略微改善表面的整体氧化还原特性,但可以通过加速 S 2 O 7 2-固有的吸热碎裂为焦硫酸铵来容易地降解 AS/ABS,同时补偿通过 Eley-Rideal 型 SCR 裂解焦硫酸铵的NH 4 +的效率适中。这可以显着提高双向的 SCR 性能-在含 SO 2的原料气流下形成齿状的含 SO A 2表面,同时促进其在低温下的长期稳定性。这些可用于发现/修改大量金属氧化物(或钒酸盐),这些金属氧化物(或钒酸盐)必须在低热能下用 SO A 2官能化或用 AS/ABS 毒化。
更新日期:2022-02-04
中文翻译:

解密含 SO2 烟道气流下单齿和双齿表面 SO32–/SO42– 改性剂促进的催化 NOX 活化和毒物破碎途径
SO A 2– ( A = 3–4; B – ) 官能团固定在金属氧化物上,该金属氧化物用于催化含 SO 2原料气流的 NH 3辅助选择性 NO X还原 (SCR) 。SO A 2-物质作为布朗斯台德酸键 (B – –H + ) 的共轭碱基和氧化还原位点 (M ( n– 1)+ –O – ) 的修饰剂,两者结合起来决定 SCR 的活性 ( − r NO X ) 和硫酸铵 (双) (AS/ABS) 毒物降解 (− r AS/ABS) 在低温下。尽管如此,它们的途径几乎没有得到澄清和探索,同时质疑单齿或双齿 SO A 2-物种在主导 - r NO X和 - r AS/ABS中的催化意义。在使用 Sb 促进的 MnV 2 O 6作为 SO A 2的储层时-提出了具有不同结合阵列、SCR 和 AS/ABS 降解的基本阶段的功能,并使用动力学控制运行与密度泛函理论计算进行了热力学评估和分析。由此得出以下结论:B – –H + •••NH 3 •••O – –M ( n –1)+与气态 NO 之间的反应阶段和 B – H 2 O /SO 2的释放阶段– •••H 2 O•••SO 2 •••H 2 O 通过解离解吸吸热并占主导地位 - r NO X和 -r AS/ABS分别作为 SCR 和 AS/ABS 退化的速率决定步骤。此外,通过提高 B – –H + •••NH 3 •••O –之间的碰撞频率,验证了单齿和双齿 SO A 2–物质在引导 - r NO X和 - r AS/ABS方面的核心作用–M ( n –1)+和 NO 并分别降低 SCR 和 AS/ABS 降解的解离 H 2 O/SO 2解吸所需的能垒。特别是单声道-齿状SO A 2-官能团可以改善表面的整体氧化还原特性,从而显着提高其在不含SO 2的原料气流下的低温SCR性能。同时,双齿 SO A 2-官能团可以略微改善表面的整体氧化还原特性,但可以通过加速 S 2 O 7 2-固有的吸热碎裂为焦硫酸铵来容易地降解 AS/ABS,同时补偿通过 Eley-Rideal 型 SCR 裂解焦硫酸铵的NH 4 +的效率适中。这可以显着提高双向的 SCR 性能-在含 SO 2的原料气流下形成齿状的含 SO A 2表面,同时促进其在低温下的长期稳定性。这些可用于发现/修改大量金属氧化物(或钒酸盐),这些金属氧化物(或钒酸盐)必须在低热能下用 SO A 2官能化或用 AS/ABS 毒化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号