当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4′-Modified Nucleosides for Antiviral Drug Discovery: Achievements and Perspectives
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-01-25 , DOI: 10.1021/acs.accounts.1c00697 Junbiao Chang 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-01-25 , DOI: 10.1021/acs.accounts.1c00697 Junbiao Chang 1, 2
Affiliation
|
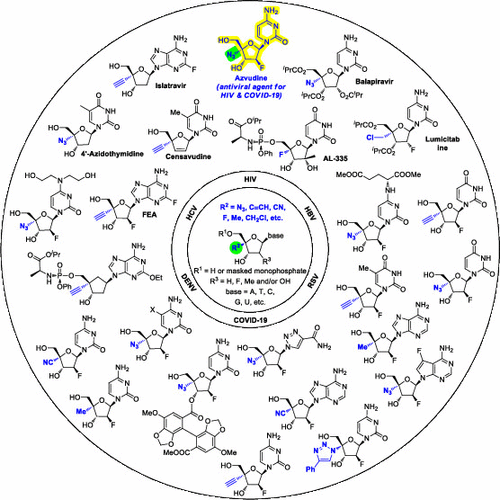
Modified nucleosides show therapeutic promise for antiviral therapies. However, issues including the emergence of drug resistance, toxicity, and coinfections have posed new challenges for nucleoside-based antiviral drug discovery, particularly in the era of the coronavirus disease 2019 (COVID-19) pandemic. Chemical manipulation could impact the antiviral potency, safety, and drug resistance of nucleosides. Generally, modified nucleosides are difficult to recognize by intracellular important enzymes as substrates and thus exhibit low toxicity. 4′-Modified nucleosides represent an important subclass of modified nucleosides for antiviral therapies. To prevent the occurrence of drug resistance, 4′-modified nucleosides should have 3′-OH, which should also be chemically unreactive for proviral DNA biosynthesis. The absence of 3′-OH may explain the occurrence of drug resistance for censavudine. The introduction of 4′-substituents improves enzymatic and acidic stability and makes the nucleosides more lipophilic, thus improving cell permeability and bioavailability. Steric hindrance between the 4′-substituent and 3′-OH changes the furanose conformation to the 3′-endo type, in which the oxygen lone pair on the furanose ring could not form an oxocarbonium ion for glycolysis. Currently, seven 4′-modified nucleoside drug candidates such as azvudine (also known as FNC), islatravir, censavudine, balapiravir, lumicitabine, AL-335, and 4-azidothymidine have progressed into clinical stages for treating viral infections. Of note, FNC was officially approved by NMPA in July 2021 for use in adult patients with high HIV-1 virus loads (nos. H20210035 and H20210036), providing an alternative therapeutic for patients with HIV-1. The long-term cellular retention of FNC suggests its potential as a long-lasting pre-exposure prophylaxis (PrEP) agent for preventing HIV-1 infection. Mechanistically, FNC not only inhibited HIV-1 reverse transcription and replication but also restored A3G expression in peripheral blood CD4+ T cells in HIV-1 patients receiving FNC. The 4′-azido group in azvudine stabilizes the 3′-C-endo (north) conformation by steric effects and the formation of an intramolecular hydrogen bond with the 3′-OH group, thus decreasing the nucleophilicity of 3′-OH. The north conformation may also enhance the phosphorylation efficiency of FNC by cellular kinases. Encouragingly, FNC, islatravir, and balapiravir show promise for the treatment of coronaviruses, of which FNC has advanced to phase 3 clinical trials in different countries to treat patients with COVID-19 (clinical trial numbers: NCT04668235 and NCT04425772). FNC cured the COVID-19 disease in almost all patients and showed better therapeutic efficacy than remdesivir. In this Account, we provide an overview of 4′-modified nucleoside analogs in clinical stages for antiviral therapies, highlighting the drug discovery strategies, structure–activity relationship studies, and preclinical/clinical studies and also give our perspectives on nucleoside-based antiviral drug discovery.
中文翻译:

用于抗病毒药物发现的 4'-修饰核苷:成就和前景
修饰的核苷显示出抗病毒疗法的治疗前景。然而,包括耐药性、毒性和共感染在内的问题对基于核苷的抗病毒药物的发现提出了新的挑战,特别是在 2019 年冠状病毒病 (COVID-19) 大流行的时代。化学操作可能会影响核苷的抗病毒效力、安全性和耐药性。一般来说,修饰的核苷很难被细胞内重要的酶识别为底物,因此毒性较低。4'-修饰核苷代表了用于抗病毒治疗的重要修饰核苷亚类。为防止耐药性的发生,4'-修饰的核苷应具有 3'-OH,其对前病毒 DNA 生物合成也应不具有化学反应性。3'-OH 的缺失可能解释了censavudine 耐药性的发生。4'-取代基的引入提高了酶和酸的稳定性,使核苷更亲脂,从而提高了细胞通透性和生物利用度。4'-取代基和3'-OH之间的空间位阻将呋喃糖构象改变为3'-远藤型,其中呋喃糖环上的氧孤对不能形成用于糖酵解的氧代碳正离子。目前,阿兹夫定(也称为 FNC)、伊拉曲韦、censavudine、巴拉匹拉韦、鲁米他滨、AL-335 和 4-叠氮胸苷等七种 4' 修饰核苷候选药物已进入临床阶段,用于治疗病毒感染。值得注意的是,FNC 于 2021 年 7 月被 NMPA 正式批准用于 HIV-1 病毒载量高的成年患者(编号 H20210035 和 H20210036),为 HIV-1 患者提供了一种替代疗法。FNC 的长期细胞保留表明其作为预防 HIV-1 感染的长效暴露前预防 (PrEP) 剂的潜力。机制上,FNC 不仅抑制 HIV-1 逆转录和复制,而且恢复外周血 CD4 中 A3G 的表达+接受 FNC 的 HIV-1 患者的 T 细胞。阿兹夫定中的 4'-叠氮基稳定了 3'-C- endo(北)通过空间效应构象和与 3'-OH 基团形成分子内氢键,从而降低 3'-OH 的亲核性。北构象还可以提高细胞激酶对 FNC 的磷酸化效率。令人鼓舞的是,FNC、islatravir 和 balapiravir 显示出治疗冠状病毒的前景,其中 FNC 已在不同国家推进到 3 期临床试验,以治疗 COVID-19 患者(临床试验编号:NCT04668235 和 NCT04425772)。FNC 治愈了几乎所有患者的 COVID-19 疾病,并显示出比瑞德西韦更好的治疗效果。在本报告中,我们概述了抗病毒治疗临床阶段的 4'-修饰核苷类似物,重点介绍了药物发现策略、构效关系研究、
更新日期:2022-01-25
中文翻译:

用于抗病毒药物发现的 4'-修饰核苷:成就和前景
修饰的核苷显示出抗病毒疗法的治疗前景。然而,包括耐药性、毒性和共感染在内的问题对基于核苷的抗病毒药物的发现提出了新的挑战,特别是在 2019 年冠状病毒病 (COVID-19) 大流行的时代。化学操作可能会影响核苷的抗病毒效力、安全性和耐药性。一般来说,修饰的核苷很难被细胞内重要的酶识别为底物,因此毒性较低。4'-修饰核苷代表了用于抗病毒治疗的重要修饰核苷亚类。为防止耐药性的发生,4'-修饰的核苷应具有 3'-OH,其对前病毒 DNA 生物合成也应不具有化学反应性。3'-OH 的缺失可能解释了censavudine 耐药性的发生。4'-取代基的引入提高了酶和酸的稳定性,使核苷更亲脂,从而提高了细胞通透性和生物利用度。4'-取代基和3'-OH之间的空间位阻将呋喃糖构象改变为3'-远藤型,其中呋喃糖环上的氧孤对不能形成用于糖酵解的氧代碳正离子。目前,阿兹夫定(也称为 FNC)、伊拉曲韦、censavudine、巴拉匹拉韦、鲁米他滨、AL-335 和 4-叠氮胸苷等七种 4' 修饰核苷候选药物已进入临床阶段,用于治疗病毒感染。值得注意的是,FNC 于 2021 年 7 月被 NMPA 正式批准用于 HIV-1 病毒载量高的成年患者(编号 H20210035 和 H20210036),为 HIV-1 患者提供了一种替代疗法。FNC 的长期细胞保留表明其作为预防 HIV-1 感染的长效暴露前预防 (PrEP) 剂的潜力。机制上,FNC 不仅抑制 HIV-1 逆转录和复制,而且恢复外周血 CD4 中 A3G 的表达+接受 FNC 的 HIV-1 患者的 T 细胞。阿兹夫定中的 4'-叠氮基稳定了 3'-C- endo(北)通过空间效应构象和与 3'-OH 基团形成分子内氢键,从而降低 3'-OH 的亲核性。北构象还可以提高细胞激酶对 FNC 的磷酸化效率。令人鼓舞的是,FNC、islatravir 和 balapiravir 显示出治疗冠状病毒的前景,其中 FNC 已在不同国家推进到 3 期临床试验,以治疗 COVID-19 患者(临床试验编号:NCT04668235 和 NCT04425772)。FNC 治愈了几乎所有患者的 COVID-19 疾病,并显示出比瑞德西韦更好的治疗效果。在本报告中,我们概述了抗病毒治疗临床阶段的 4'-修饰核苷类似物,重点介绍了药物发现策略、构效关系研究、





























 京公网安备 11010802027423号
京公网安备 11010802027423号