当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclic vs. acyclic alkyne towards Hg2+ ion detection: combined experimental and theoretical studies
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-01-11 , DOI: 10.1039/d1nj05707c Adwitiya Pal 1 , Bappaditya Goswami 2 , Arunabha Thakur 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2022-01-11 , DOI: 10.1039/d1nj05707c Adwitiya Pal 1 , Bappaditya Goswami 2 , Arunabha Thakur 1
Affiliation
|
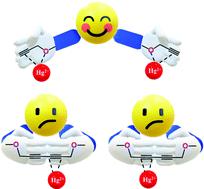
Alkynes constitute a versatile class of functional groups, which can potentially undergo a variety of transformations in organic synthesis. The interaction between the alkyne unit and Hg2+ ion has been well-known for several decades. However, with the aim to investigate this interaction in more detail, we have designed and synthesized two thermally stable probes, 3 and 4. Probe 3 is an acyclic compound containing two terminal alkyne units, whereas probe 4 is a cyclic molecule with an internally conjugated C2-symmetric 1,3-dialkyne unit. Interestingly, both receptors possess alkyne units but reacted differently with Hg2+ ions. The acyclic probe 3 with two terminal alkynes interacted with two Hg2+ ions, whereas cyclic probe 4, with a cage-like structure, interacted with only one Hg2+ ion. The Hg2+ ion could only interact with one alkyne unit in probe 4 to avoid steric repulsion between two Hg2+ ions. The differences in the selective responses of the cyclic and acyclic structures towards Hg2+ ions were thoroughly established via photophysical (UV-vis and emission spectroscopy) and electrochemical analysis together with theoretical (DFT) studies. The probable binding sites of the Hg2+ ion with synthesized probes were determined by 1H NMR and IR titrations, which indicated that the terminal and conjugated di-alkyne units interact with the Hg2+ ion via a favorable soft-soft interaction. Furthermore, the sweeping motion of the Hg2+ ion between the two alkyne units of the 1,3-dialkyne moiety in 4 was also confirmed by DFT calculations. Unlike the Hg2+ ion, Cu2+ and Fe3+ ions did not interact with the probes, rather they induced oxidation of the ferrocene centre. Both receptors 3 and 4 and their corresponding metal complexes, [3·2Hg2+] and [4·Hg2+], respectively, were stable in the physiological pH range (pH around 7) and thermally stable up to 60 °C. For the first time, the present study focused on the comparative responses of acyclic and cyclic architectures of the same molecular unit towards metal ion recognition, supporting the fact that the alkyne group in different environments behaves differently with the same soft metal.
中文翻译:

环状与非环状炔烃对 Hg2+ 离子检测:结合实验和理论研究
炔烃构成了一类多功能的官能团,在有机合成中可能会发生各种转变。几十年来,炔烃单元与Hg 2+离子之间的相互作用已为人们所熟知。然而,为了更详细地研究这种相互作用,我们设计并合成了两种热稳定探针3和4。探针3是含有两个末端炔烃单元的无环化合物,而探针4是具有内部共轭C 2 -对称1,3-二炔烃单元的环状分子。有趣的是,这两种受体都具有炔烃单元,但与 Hg 2+离子的反应不同。非循环探针具有两个末端炔烃的3与两个Hg 2+离子相互作用,而具有笼状结构的环状探针4仅与一个Hg 2+离子相互作用。Hg 2+离子只能与探针4中的一个炔烃单元相互作用,以避免两个Hg 2+离子之间的空间排斥。通过光物理(UV-vis 和发射光谱)和电化学分析以及理论(DFT)研究,彻底确定了环状和非环状结构对 Hg 2+离子的选择性响应差异。Hg 2+离子与合成探针的可能结合位点由下式确定1 H NMR和IR滴定表明,末端和共轭二炔单元与Hg 2+离子通过有利的软-软相互作用发生相互作用。此外, 4中1,3-二炔部分的两个炔单元之间的Hg 2+离子的扫掠运动也通过DFT计算得到证实。与Hg 2+离子不同,Cu 2+和Fe 3+离子不与探针相互作用,而是诱导二茂铁中心的氧化。受体3和4及其相应的金属络合物 [ 3 ·2Hg 2+ ] 和 [ 4 ·Hg2+ ] 分别在生理 pH 值范围内(pH 值约为 7)和高达 60 °C 的热稳定性。本研究首次关注同一分子单元的无环和环状结构对金属离子识别的比较响应,支持了不同环境中的炔基对相同软金属表现不同的事实。
更新日期:2022-01-11
中文翻译:

环状与非环状炔烃对 Hg2+ 离子检测:结合实验和理论研究
炔烃构成了一类多功能的官能团,在有机合成中可能会发生各种转变。几十年来,炔烃单元与Hg 2+离子之间的相互作用已为人们所熟知。然而,为了更详细地研究这种相互作用,我们设计并合成了两种热稳定探针3和4。探针3是含有两个末端炔烃单元的无环化合物,而探针4是具有内部共轭C 2 -对称1,3-二炔烃单元的环状分子。有趣的是,这两种受体都具有炔烃单元,但与 Hg 2+离子的反应不同。非循环探针具有两个末端炔烃的3与两个Hg 2+离子相互作用,而具有笼状结构的环状探针4仅与一个Hg 2+离子相互作用。Hg 2+离子只能与探针4中的一个炔烃单元相互作用,以避免两个Hg 2+离子之间的空间排斥。通过光物理(UV-vis 和发射光谱)和电化学分析以及理论(DFT)研究,彻底确定了环状和非环状结构对 Hg 2+离子的选择性响应差异。Hg 2+离子与合成探针的可能结合位点由下式确定1 H NMR和IR滴定表明,末端和共轭二炔单元与Hg 2+离子通过有利的软-软相互作用发生相互作用。此外, 4中1,3-二炔部分的两个炔单元之间的Hg 2+离子的扫掠运动也通过DFT计算得到证实。与Hg 2+离子不同,Cu 2+和Fe 3+离子不与探针相互作用,而是诱导二茂铁中心的氧化。受体3和4及其相应的金属络合物 [ 3 ·2Hg 2+ ] 和 [ 4 ·Hg2+ ] 分别在生理 pH 值范围内(pH 值约为 7)和高达 60 °C 的热稳定性。本研究首次关注同一分子单元的无环和环状结构对金属离子识别的比较响应,支持了不同环境中的炔基对相同软金属表现不同的事实。































 京公网安备 11010802027423号
京公网安备 11010802027423号