当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile preparation of dihydro-1,4-benzothiazine derivatives via oxidative ring-expansion of 2-aminobenzothiazoles with olefins
Chemical Communications ( IF 4.3 ) Pub Date : 2022-01-19 , DOI: 10.1039/d1cc06756g
Qing Sun 1 , Xiaoguang Bao 1
Chemical Communications ( IF 4.3 ) Pub Date : 2022-01-19 , DOI: 10.1039/d1cc06756g
Qing Sun 1 , Xiaoguang Bao 1
Affiliation
|
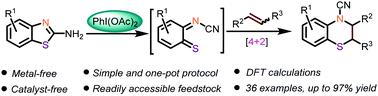
A concise and efficient approach to prepare dihydro-1,4-benzothiazine derivatives is described via oxidative ring-expansion of 2-aminobenzothiazoles with olefins under metal-free conditions. This protocol is applicable for a wide range of readily accessible 2-aminobenzothiazoles and olefins with moderate-to-good yields. The [4+2] heteroannulation between the intermediacy of oxidative ring-opening of 2-aminobenzothiazoles and olefins is suggested to rationalize the formation of the product.
中文翻译:

2-氨基苯并噻唑与烯烃氧化扩环制备二氢-1,4-苯并噻嗪衍生物
描述了一种在无金属条件下通过2-氨基苯并噻唑与烯烃的氧化扩环制备二氢 1,4-苯并噻嗪衍生物的简洁有效的方法。该协议适用于各种容易获得的 2-氨基苯并噻唑和烯烃,产率中等至良好。建议 2-氨基苯并噻唑和烯烃的氧化开环中间体之间的 [4+2] 杂环化使产物的形成合理化。
更新日期:2022-01-19
中文翻译:

2-氨基苯并噻唑与烯烃氧化扩环制备二氢-1,4-苯并噻嗪衍生物
描述了一种在无金属条件下通过2-氨基苯并噻唑与烯烃的氧化扩环制备二氢 1,4-苯并噻嗪衍生物的简洁有效的方法。该协议适用于各种容易获得的 2-氨基苯并噻唑和烯烃,产率中等至良好。建议 2-氨基苯并噻唑和烯烃的氧化开环中间体之间的 [4+2] 杂环化使产物的形成合理化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号