Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2022-01-22 , DOI: 10.1016/j.bmcl.2022.128582 Ting-Jian Zhang 1 , Yi Zhang 1 , Zhen-Hao Zhang 1 , Zhao-Ran Wang 1 , Xu Zhang 1 , Sen-Sen Hu 1 , Peng-Fei Lu 1 , Shuai Guo 1 , Fan-Hao Meng 1
|
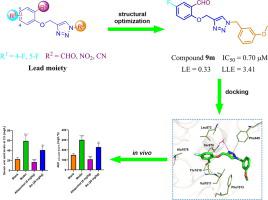
A series of 4-(phenoxymethyl)-1H-1,2,3-triazole derivatives were designed, synthesized, and evaluated for their xanthine oxidase (XO) inhibitory activities. Among these compounds, 9m emerged as the most effective XO inhibitor with an IC50 value of 0.70 μM, which was approximately 14-fold more potent than allopurinol. Additionally, compound 9m displayed favorable drug-like properties with ligand efficiency (LE) and lipophilic ligand efficiency (LLE) values of 0.33 and 3.41, respectively. We further explored the binding mode of 9m in complex with XO by molecular docking and molecular dynamics studies. In vivo hypouricemic studies also suggested that 9m could effectively lower the serum uric acid levels of rat. In summary, compound 9m could be a promising lead for further development of XO inhibitors.
中文翻译:

4-(苯氧基甲基)-1H-1,2,3-三唑衍生物作为新型黄嘌呤氧化酶抑制剂的发现
设计、合成了一系列 4-(苯氧基甲基)-1 H -1,2,3-三唑衍生物,并评估了它们的黄嘌呤氧化酶 (XO) 抑制活性。在这些化合物中,9m成为最有效的 XO 抑制剂,其 IC 50值为 0.70 μM,比别嘌呤醇强约 14 倍。此外,化合物9m表现出良好的药物样特性,配体效率 (LE) 和亲脂性配体效率 (LLE) 值分别为 0.33 和 3.41。我们通过分子对接和分子动力学研究进一步探索了9m与XO复合物的结合模式。体内降尿酸研究也表明9m能有效降低大鼠血清尿酸水平。总之,化合物9m可能是进一步开发 XO 抑制剂的有希望的先导。






























 京公网安备 11010802027423号
京公网安备 11010802027423号