International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2022-01-22 , DOI: 10.1016/j.ijhydene.2022.01.037 Siye Lv 1 , Junqi Li 1 , Beiyi Zhang 1 , Yunli Shi 1 , Xuan Liu 1 , Ting Wang 1
|
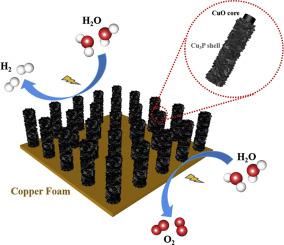
To fulfill the growing demand for green H2 fuels, low-cost, efficient, and stable bifunctional electrocatalysts must be developed. Herein, a hierarchical CuO@Cu3P/CF nanowire core-shell heterostructure with transferable active centers was developed for a bifunctional electrocatalyst with high activity. In this system, the transfer of electrochemically active centers between Cu3P and CuO is used to facilitate the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), respectively. Particularly, Cu3P acts as the active center for HER, while the active center shifts to CuO for OER reaction, and Cu3P acts as a co-catalyst to improve the conductivity of the system. Benefit from superhydrophilicity's high electrochemical surface area and synergistic effect of CuO core and Cu3P shell, and CuO@Cu3P/CF shown significant catalytic activity for hydrogen or oxygen evolution, requiring low overpotentials of 144 and 267 mV to achieve a current density of 10 mA cm−2. In addition, the assembled CuO@Cu3P/CF-based electrolyzer exhibit excellent overall water splitting performance with a low operating voltage of 1.75 V at 10 mA cm−2 and a negligible decrease in catalytic activity. This gives encouraging evidence for the utility of our catalysts in application areas.
中文翻译:

在泡沫铜基底上原位生长具有可转移活性中心的分级CuO@Cu3P异质结构作为碱性介质中全水分解的双功能电催化剂
为了满足对绿色 H 2燃料日益增长的需求,必须开发低成本、高效、稳定的双功能电催化剂。在此,开发了一种具有可转移活性中心的分级CuO@Cu 3 P/CF纳米线核壳异质结构,用于具有高活性的双功能电催化剂。在该系统中,Cu 3 P 和 CuO之间电化学活性中心的转移分别用于促进析氢反应 (HER) 和析氧反应 (OER)。特别是,Cu 3 P 充当 HER 的活性中心,而 OER 反应的活性中心转移到 CuO,而 Cu 3P充当助催化剂以提高系统的电导率。得益于超亲水性的高电化学表面积以及 CuO 核和 Cu 3 P 壳的协同效应,CuO@Cu 3 P/CF 对析氢或析氧表现出显着的催化活性,需要 144 和 267 mV 的低过电位才能达到电流密度10 mA cm -2。此外,组装的 CuO@Cu 3 P/CF 基电解槽表现出优异的整体水分解性能,在 10 mA cm -2下具有 1.75 V 的低工作电压和可忽略不计的催化活性降低。这为我们的催化剂在应用领域的实用性提供了令人鼓舞的证据。

































 京公网安备 11010802027423号
京公网安备 11010802027423号