当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mucoid Coating Provides a Growth Advantage to Pseudomonas aeruginosa at Oil–Water Interfaces
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2022-01-22 , DOI: 10.1021/acsabm.1c01198 Sricharani Rao Balmuri 1 , Vienvilay Phandanouvong-Lozano 1 , Stephen D. House 2 , Judith C. Yang 3 , Tagbo H.R. Niepa 4
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2022-01-22 , DOI: 10.1021/acsabm.1c01198 Sricharani Rao Balmuri 1 , Vienvilay Phandanouvong-Lozano 1 , Stephen D. House 2 , Judith C. Yang 3 , Tagbo H.R. Niepa 4
Affiliation
|
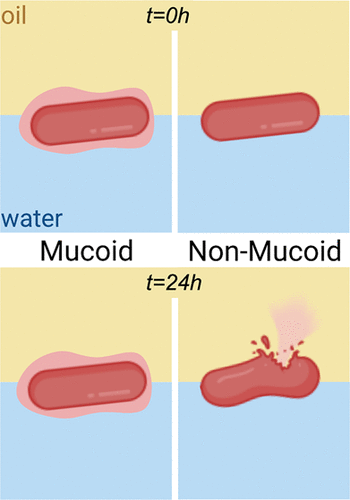
Chronic lung infection with bacterial biofilms is one of the leading causes of death in cystic fibrosis (CF) patients. Among many species infecting the lung airways, Pseudomonas aeruginosa is the major pathogen colonizing and persisting throughout the patient’s life. The microorganism undergoes pathoadaptation, while switching from a nonmucoid to a mucoid phenotype, improving the mechanical properties of the resulting biofilms. Previous investigation of the dynamic rheological properties of nonmucoid (PANT) and mucoid (PASL) clinical P. aeruginosa isolates exposed to interfacial stresses demonstrated that the mucoid strains formed films with stronger resistance to bending and nonlinear relaxation to compression and tension. We hypothesize that the mucoid switch provides a growth advantage to P. aeruginosa through the development of interfacial films with viscoelastic properties enabling cell survival. Here, we investigate the physiological response of the mucoid and the nonmucoid P. aeruginosa to interfacial entrapment. Our results, both macroscopic and molecular, reveal that mucoid coating plays an important role in protecting the bacteria from interfacial stresses. Cell characterizations using electron and fluorescence microscopies showed higher proportion of dead nonmucoid cells compared to mucoid cells on interfacial exposure. For example, scanning transmission electron microscopy (STEM) imaging showed that 96.6% of nonmucoid cells vs only 22.2% of mucoid cells were lysed owing to interfacial stress. Furthermore, the transcriptional profiling of P. aeruginosa cells indicated the upregulation of pel, psl, and alginate genes encoding for exopolysaccharide biomaterials is associated with mucoid cells’ ability to cope with the interfacial environments. Further characterization of real-time gene regulation at interfaces will elucidate the effects of interfacial environment on the regulation of bacterial virulence.
中文翻译:

粘液涂层在油水界面为铜绿假单胞菌提供生长优势
细菌生物膜引起的慢性肺部感染是囊性纤维化 (CF) 患者死亡的主要原因之一。在许多感染肺气道的物种中,铜绿假单胞菌是在患者一生中定植和持续存在的主要病原体。微生物经历了病理适应,同时从非粘液表型转变为粘液表型,从而改善了所得生物膜的机械性能。先前对非粘液 (PANT) 和粘液 (PASL) 临床铜绿假单胞菌的动态流变学特性的研究暴露于界面应力的分离株表明,粘液应变形成的薄膜具有更强的抗弯曲性和对压缩和拉伸的非线性松弛。我们假设粘液开关通过开发具有粘弹性特性的界面膜为铜绿假单胞菌提供生长优势,从而使细胞存活。在这里,我们研究了粘液和非粘液铜绿假单胞菌的生理反应到界面截留。我们的宏观和分子结果表明,粘液涂层在保护细菌免受界面应力方面起着重要作用。使用电子和荧光显微镜进行的细胞表征显示,与界面暴露的粘液细胞相比,死亡的非粘液细胞比例更高。例如,扫描透射电子显微镜 (STEM) 成像显示,96.6% 的非粘液细胞与只有 22.2% 的粘液细胞由于界面应力而被裂解。此外,铜绿假单胞菌的转录谱细胞表明,编码胞外多糖生物材料的 pel、psl 和藻酸盐基因的上调与粘液细胞应对界面环境的能力有关。界面实时基因调控的进一步表征将阐明界面环境对细菌毒力调控的影响。
更新日期:2022-01-22
中文翻译:

粘液涂层在油水界面为铜绿假单胞菌提供生长优势
细菌生物膜引起的慢性肺部感染是囊性纤维化 (CF) 患者死亡的主要原因之一。在许多感染肺气道的物种中,铜绿假单胞菌是在患者一生中定植和持续存在的主要病原体。微生物经历了病理适应,同时从非粘液表型转变为粘液表型,从而改善了所得生物膜的机械性能。先前对非粘液 (PANT) 和粘液 (PASL) 临床铜绿假单胞菌的动态流变学特性的研究暴露于界面应力的分离株表明,粘液应变形成的薄膜具有更强的抗弯曲性和对压缩和拉伸的非线性松弛。我们假设粘液开关通过开发具有粘弹性特性的界面膜为铜绿假单胞菌提供生长优势,从而使细胞存活。在这里,我们研究了粘液和非粘液铜绿假单胞菌的生理反应到界面截留。我们的宏观和分子结果表明,粘液涂层在保护细菌免受界面应力方面起着重要作用。使用电子和荧光显微镜进行的细胞表征显示,与界面暴露的粘液细胞相比,死亡的非粘液细胞比例更高。例如,扫描透射电子显微镜 (STEM) 成像显示,96.6% 的非粘液细胞与只有 22.2% 的粘液细胞由于界面应力而被裂解。此外,铜绿假单胞菌的转录谱细胞表明,编码胞外多糖生物材料的 pel、psl 和藻酸盐基因的上调与粘液细胞应对界面环境的能力有关。界面实时基因调控的进一步表征将阐明界面环境对细菌毒力调控的影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号