Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of a New Solvent Co-Catalyzed Mechanism in Heterogeneous Catalysis: A First-Principles Study with Molecular Dynamics on Acetaldehyde Hydrogenation on Birnessite
JACS Au ( IF 8.5 ) Pub Date : 2022-01-21 , DOI: 10.1021/jacsau.1c00452
Wenbo Xie 1 , Glenn Reid 1 , P Hu 1
JACS Au ( IF 8.5 ) Pub Date : 2022-01-21 , DOI: 10.1021/jacsau.1c00452
Wenbo Xie 1 , Glenn Reid 1 , P Hu 1
Affiliation
|
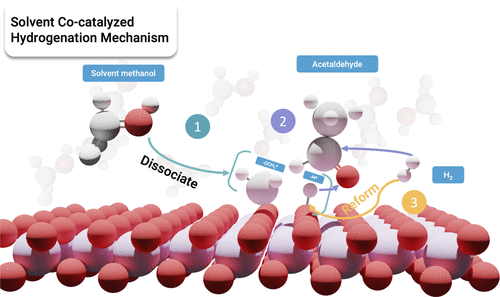
Heterogenous hydrogenation reactions are essential in a wide range of chemical industries. In this work, we find that the hydrogenation of acetaldehyde on birnessite cannot occur through the traditional mechanisms due to the strong adsorption of the aldehyde and hydrogen on the surface, using first-principles calculations. We discover that this reaction can occur feasibly via a solvent-cocatalyzed mechanism with molecular hydrogen in the liquid phase: a methanol solvent or a similar solvent is required for the reaction. Free energy calculations shows that the methanol solvent preferentially fills the oxygen vacancies of the catalyst surface and spontaneously dissociates on the surface, in which the resulting hydroxyl group then acts as the coordination site for the carbonyl bond and allows the reaction to proceed without adsorption of the reactants on the surface. The reasons this new mechanism is more favorable over the traditional mechanisms in the literature are scrutinized and discussed. The new mechanism may be followed in many other systems.
中文翻译:

多相催化中新的溶剂共催化机制的发现:水钠沸石上乙醛加氢的分子动力学第一性原理研究
多相加氢反应在广泛的化学工业中是必不可少的。在这项工作中,我们使用第一性原理计算发现,由于醛和氢在表面上的强吸附,乙醛在水钠锰矿上的氢化不能通过传统机制发生。我们发现该反应可以通过溶剂助催化机制与液相中的分子氢发生:反应需要甲醇溶剂或类似溶剂。自由能计算表明,甲醇溶剂优先填充催化剂表面的氧空位并在表面自发解离,其中产生的羟基然后充当羰基键的配位点,并允许反应进行而不吸附反应物在表面上。这种新机制比文献中的传统机制更有利的原因被仔细审查和讨论。许多其他系统可能会遵循新机制。
更新日期:2022-01-21
中文翻译:

多相催化中新的溶剂共催化机制的发现:水钠沸石上乙醛加氢的分子动力学第一性原理研究
多相加氢反应在广泛的化学工业中是必不可少的。在这项工作中,我们使用第一性原理计算发现,由于醛和氢在表面上的强吸附,乙醛在水钠锰矿上的氢化不能通过传统机制发生。我们发现该反应可以通过溶剂助催化机制与液相中的分子氢发生:反应需要甲醇溶剂或类似溶剂。自由能计算表明,甲醇溶剂优先填充催化剂表面的氧空位并在表面自发解离,其中产生的羟基然后充当羰基键的配位点,并允许反应进行而不吸附反应物在表面上。这种新机制比文献中的传统机制更有利的原因被仔细审查和讨论。许多其他系统可能会遵循新机制。




































 京公网安备 11010802027423号
京公网安备 11010802027423号