当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concise Process for Pyriftalid Synthesis by Introducing the Mercapto Group Directly from a Nitro Group
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.oprd.1c00384 Zhong Li 1 , Yong-Jian Chi 1 , Yi-Wen Huang 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.oprd.1c00384 Zhong Li 1 , Yong-Jian Chi 1 , Yi-Wen Huang 2
Affiliation
|
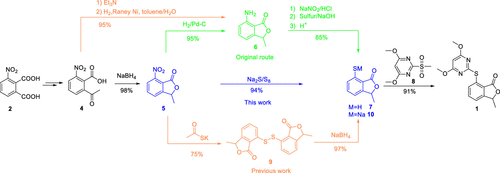
A concise and efficient process for the synthesis of pyriftalid is described herein. The improved process features the direct introduction of the mercapto group by one-step substitution of a nitro group using sodium sulfide and elemental sulfur. A systematic study of the key step is also carried out in this article. Compared with previous routes, the optimized route is shorter and avoids a hazardous catalytic reduction and a subsequent diazotization reaction. Salt waste generated in workup is reduced as well. Pyriftalid is obtained in 68% overall yield and 99.88% purity in five steps with 3-nitrophthalic acid as the starting material.
中文翻译:

直接从硝基基团中引入巯基的 Pyriftalid 合成简明工艺
本文描述了一种用于合成 pyriftalid 的简明有效的方法。改进的方法的特点是通过使用硫化钠和元素硫一步取代硝基来直接引入巯基。本文还对关键步骤进行了系统研究。与以前的路线相比,优化路线更短,避免了危险的催化还原和随后的重氮化反应。后处理中产生的盐废物也减少了。以 3-硝基邻苯二甲酸为原料,分五步获得 Pyriftalid,总产率为 68%,纯度为 99.88%。
更新日期:2022-01-21
中文翻译:

直接从硝基基团中引入巯基的 Pyriftalid 合成简明工艺
本文描述了一种用于合成 pyriftalid 的简明有效的方法。改进的方法的特点是通过使用硫化钠和元素硫一步取代硝基来直接引入巯基。本文还对关键步骤进行了系统研究。与以前的路线相比,优化路线更短,避免了危险的催化还原和随后的重氮化反应。后处理中产生的盐废物也减少了。以 3-硝基邻苯二甲酸为原料,分五步获得 Pyriftalid,总产率为 68%,纯度为 99.88%。































 京公网安备 11010802027423号
京公网安备 11010802027423号