当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perylene Bisimide Cyclophanes as Biaryl Enantiomerization Catalysts─Explorations into π–π Catalysis and Host–Guest Chirality Transfer
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.joc.1c02719 Asja A Kroeger 1 , Amir Karton 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-21 , DOI: 10.1021/acs.joc.1c02719 Asja A Kroeger 1 , Amir Karton 1
Affiliation
|
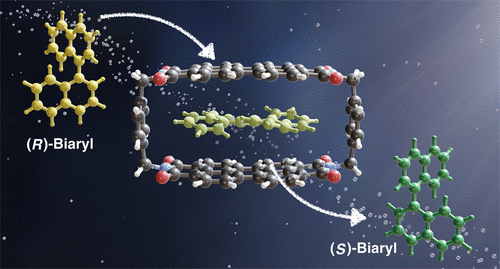
The racemization of axially chiral biaryls is a fundamental step toward transforming kinetic resolutions into dynamic kinetic resolutions (DKRs). The high enantiomerization barriers of many biaryl compounds of synthetic relevance, however, may render DKR strategies challenging. Here, we computationally explore the potential of a para-xylene bridged perylene bisimide cyclophane to serve as a conceptually transferrable biaryl enantiomerization catalyst for fundamental biphenyl and binaphthyl scaffolds, as well as the versatile reagent 1,1′-binaphthyl-2,2′-diol and a precursor to the heterobiaryl ligand QUINAP. The calculated enantiomerization barriers of the different biaryls decrease by 19.8–73.2% upon complexation, suggesting that the cyclophane may form an effective biaryl racemization catalyst. We find that these observed barrier reductions predominantly originate from a combination of transition structure stabilization through π–π stacking interactions between the shape-complementary transition structures and catalyst, as well as ground-state destabilization of the less complementary reactants, indicating a generalizable strategy toward biaryl racemization catalysis. In exploring all enantiomerization pathways of the biaryls under consideration, we further find a systematic enantiomer- and conformer-dependent chirality transfer from biaryl to cyclophane in host–guest complexes.
中文翻译:

苝双酰亚胺环芳烃作为联芳基对映异构化催化剂─π-π催化和主客体手性转移的探索
轴向手性联芳基的外消旋化是将动力学分辨率转化为动态动力学分辨率 (DKR) 的基本步骤。然而,许多与合成相关的联芳基化合物的高对映异构化障碍可能使 DKR 策略具有挑战性。在这里,我们通过计算探索para-二甲苯桥联苝双酰亚胺环烷可用作基本联苯和联萘支架的概念上可转移的联芳基对映异构化催化剂,以及多功能试剂 1,1'-binaphthyl-2,2'-diol 和杂联芳基配体 QUINAP 的前体。计算得到的不同联芳基的对映异构化势垒在络合后降低了 19.8-73.2%,这表明环芳可以形成有效的联芳基外消旋催化剂。我们发现,这些观察到的势垒减少主要源于通过形状互补过渡结构和催化剂之间的 π-π 堆积相互作用实现的过渡结构稳定化,以及互补性较低的反应物的基态去稳定化,这表明了一种可推广的策略联芳基消旋催化。
更新日期:2022-01-21
中文翻译:

苝双酰亚胺环芳烃作为联芳基对映异构化催化剂─π-π催化和主客体手性转移的探索
轴向手性联芳基的外消旋化是将动力学分辨率转化为动态动力学分辨率 (DKR) 的基本步骤。然而,许多与合成相关的联芳基化合物的高对映异构化障碍可能使 DKR 策略具有挑战性。在这里,我们通过计算探索para-二甲苯桥联苝双酰亚胺环烷可用作基本联苯和联萘支架的概念上可转移的联芳基对映异构化催化剂,以及多功能试剂 1,1'-binaphthyl-2,2'-diol 和杂联芳基配体 QUINAP 的前体。计算得到的不同联芳基的对映异构化势垒在络合后降低了 19.8-73.2%,这表明环芳可以形成有效的联芳基外消旋催化剂。我们发现,这些观察到的势垒减少主要源于通过形状互补过渡结构和催化剂之间的 π-π 堆积相互作用实现的过渡结构稳定化,以及互补性较低的反应物的基态去稳定化,这表明了一种可推广的策略联芳基消旋催化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号