当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contact Ion Pair Formation Is Not Necessarily Stronger than Solvent Shared Ion Pairing
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jpclett.1c03576 Kenneth D Judd 1 , Nicole M Gonzalez 1 , Tinglu Yang 1 , Paul S Cremer 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.jpclett.1c03576 Kenneth D Judd 1 , Nicole M Gonzalez 1 , Tinglu Yang 1 , Paul S Cremer 1, 2
Affiliation
|
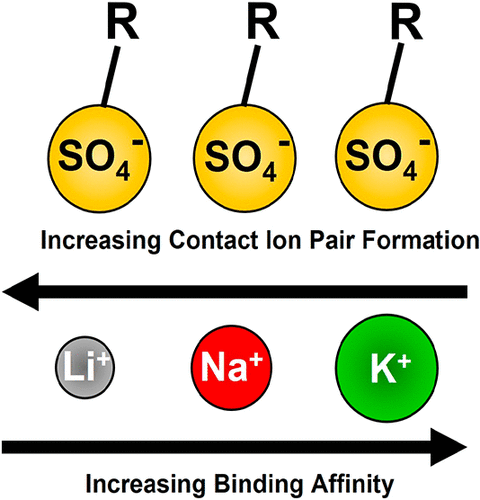
Vibrational sum frequency spectroscopy (VSFS) and pressure–area Langmuir trough measurements were used to investigate the binding of alkali metal cations to eicosyl sulfate (ESO4) surfactants in monolayers at the air/water interface. The number density of sulfate groups could be tuned by mixing the anionic surfactant with eicosanol. The equilibrium dissociation constant for K+ to the fatty sulfate interface showed 10 times greater affinity than for Li+ and approximately 3 times greater than for Na+. All three cations formed solvent shared ion pairs when the mole fraction of ESO4 was 0.33 or lower. Above this threshold charge density, Li+ formed contact ion pairs with the sulfate headgroups, presumably via bridging structures. By contrast, K+ only bound to the sulfate moieties in solvent shared ion pairing configurations. The behavior for Na+ was intermediate. These results demonstrate that there is not necessarily a correlation between contact ion pair formation and stronger binding affinity.
中文翻译:

接触离子对的形成不一定比溶剂共享离子对强
振动和频谱 (VSFS) 和压力面积朗缪尔槽测量用于研究碱金属阳离子与空气/水界面单层中的二十烷基硫酸盐 (ESO 4 ) 表面活性剂的结合。硫酸盐基团的数量密度可以通过将阴离子表面活性剂与二十烷醇混合来调节。K +与脂肪硫酸盐界面的平衡解离常数显示出比 Li +大 10 倍的亲和力,比 Na +大约 3 倍。当 ESO 4的摩尔分数为 0.33 或更低时,所有三种阳离子形成溶剂共享离子对。高于该阈值电荷密度,Li +与硫酸盐头基形成接触离子对,可能是通过桥接结构。相比之下,K +仅与溶剂共享离子配对配置中的硫酸盐部分结合。Na +的行为是中等的。这些结果表明,接触离子对的形成与更强的结合亲和力之间不一定存在相关性。
更新日期:2022-01-27
中文翻译:

接触离子对的形成不一定比溶剂共享离子对强
振动和频谱 (VSFS) 和压力面积朗缪尔槽测量用于研究碱金属阳离子与空气/水界面单层中的二十烷基硫酸盐 (ESO 4 ) 表面活性剂的结合。硫酸盐基团的数量密度可以通过将阴离子表面活性剂与二十烷醇混合来调节。K +与脂肪硫酸盐界面的平衡解离常数显示出比 Li +大 10 倍的亲和力,比 Na +大约 3 倍。当 ESO 4的摩尔分数为 0.33 或更低时,所有三种阳离子形成溶剂共享离子对。高于该阈值电荷密度,Li +与硫酸盐头基形成接触离子对,可能是通过桥接结构。相比之下,K +仅与溶剂共享离子配对配置中的硫酸盐部分结合。Na +的行为是中等的。这些结果表明,接触离子对的形成与更强的结合亲和力之间不一定存在相关性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号