当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Practical and Greener Process for the Dual Leucine Zipper Kinase Inhibitor GDC-0134 Comprising Two SNAr Reactions, Oxidation and Suzuki Coupling
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.oprd.1c00389 Fabienne Hoffmann-Emery 1 , Katrin Niedermann 1 , Pankaj D. Rege 1 , Manuel Konrath 1 , Christian Lautz 1 , Anne Katrin Kraft 1 , Carine Steiner 1 , Fritz Bliss 1 , André Hell 1 , Rolf Fischer 1 , Diane E. Carrera 2 , Danial Beaudry 2 , Remy Angelaud 2 , Sushant Malhotra 2 , Francis Gosselin 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.oprd.1c00389 Fabienne Hoffmann-Emery 1 , Katrin Niedermann 1 , Pankaj D. Rege 1 , Manuel Konrath 1 , Christian Lautz 1 , Anne Katrin Kraft 1 , Carine Steiner 1 , Fritz Bliss 1 , André Hell 1 , Rolf Fischer 1 , Diane E. Carrera 2 , Danial Beaudry 2 , Remy Angelaud 2 , Sushant Malhotra 2 , Francis Gosselin 2
Affiliation
|
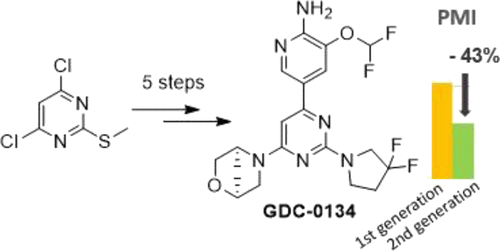
A sustainable second-generation process for GDC-0134 was developed with a particular focus on safety and greenness, while complying with strong specifications to supply pivotal clinical studies. Through these efforts, we discovered solvents to replace the solvents classified as substances of very high concern by the REACH regulation in two SNAr steps. We further established a safer and faster way to oxidize the sulfanyl to the sulfonyl intermediate by dosing H2O2 at higher temperatures, reducing accumulation while minimizing uncontrolled H2O2 decomposition. The reaction conditions for the Suzuki coupling and the second SNAr were modified to increase the selectivity in these two steps. A change in the solvent for the final crystallization allowed operation at higher concentrations and delivery of a highly pure active pharmaceutical ingredient (API). The new improved process reduced the process mass intensity by approximately 40% and was successfully used to produce 150 kg of GDC-0134.
中文翻译:

开发一种实用且更环保的双亮氨酸拉链激酶抑制剂 GDC-0134 工艺,包括两个 SNAr 反应、氧化和 Suzuki 偶联
为 GDC-0134 开发了可持续的第二代工艺,特别关注安全性和绿色性,同时遵守严格的规范以提供关键的临床研究。通过这些努力,我们发现溶剂可在两个 S N Ar 步骤中替代 REACH 法规中列为高度关注物质的溶剂。我们进一步建立了一种更安全、更快速的方法,通过在更高温度下投加 H 2 O 2将硫烷基氧化为磺酰基中间体,从而减少积累,同时最大限度地减少不受控制的 H 2 O 2分解。Suzuki 偶联反应和第二次 S N的反应条件Ar 被修改以增加这两个步骤中的选择性。最终结晶溶剂的变化允许在更高浓度下操作并提供高纯度的活性药物成分 (API)。新的改进工艺将工艺质量强度降低了约 40%,并成功用于生产 150 公斤的 GDC-0134。
更新日期:2022-01-20
中文翻译:

开发一种实用且更环保的双亮氨酸拉链激酶抑制剂 GDC-0134 工艺,包括两个 SNAr 反应、氧化和 Suzuki 偶联
为 GDC-0134 开发了可持续的第二代工艺,特别关注安全性和绿色性,同时遵守严格的规范以提供关键的临床研究。通过这些努力,我们发现溶剂可在两个 S N Ar 步骤中替代 REACH 法规中列为高度关注物质的溶剂。我们进一步建立了一种更安全、更快速的方法,通过在更高温度下投加 H 2 O 2将硫烷基氧化为磺酰基中间体,从而减少积累,同时最大限度地减少不受控制的 H 2 O 2分解。Suzuki 偶联反应和第二次 S N的反应条件Ar 被修改以增加这两个步骤中的选择性。最终结晶溶剂的变化允许在更高浓度下操作并提供高纯度的活性药物成分 (API)。新的改进工艺将工艺质量强度降低了约 40%,并成功用于生产 150 公斤的 GDC-0134。






























 京公网安备 11010802027423号
京公网安备 11010802027423号