当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Initial Route Scouting and Final Process Development for the Multi-Kg Production of 3-Fluoro-6-methoxyquinoline from p-Anisidine and 2-Fluoromalonic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.oprd.1c00414 Gabriel Schäfer 1 , Tony Fleischer 1 , Nicole Blumer 1 , Megan Udry 1 , Stefan Reber 1 , Ian Stansfield 2 , Yuanhua Liu 2 , Yan Li 2 , Pixu Li 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-01-20 , DOI: 10.1021/acs.oprd.1c00414 Gabriel Schäfer 1 , Tony Fleischer 1 , Nicole Blumer 1 , Megan Udry 1 , Stefan Reber 1 , Ian Stansfield 2 , Yuanhua Liu 2 , Yan Li 2 , Pixu Li 2
Affiliation
|
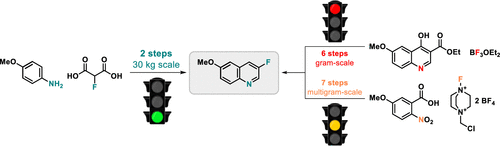
A scalable route to 3-fluoro-6-methoxyquinoline needed to be developed as multi-kg amounts of this heterocycle were required. Initial route development focused on the formation of the key C–F bond via a Balz–Schiemann reaction or electrophilic fluorination using Selectfluor. Both routes were developed on laboratory scale and provided gram amounts of 3-fluoro-6-methoxyquinoline. However, due to process safety concerns and high step counts, both routes were not suitable for further scale up. Therefore, a third approach was developed, in which the desired heterocycle was formed via condensation of p-anisidine with 2-fluoromalonic acid, two inexpensive and commercially available starting materials. After intensive optimization and safety studies, this POCl3-mediated process was successfully scaled up to a 32 kg scale. After final hydrodechlorination, 12 kg of 3-fluoro-6-methoxyquinoline with excellent purity was produced.
中文翻译:

从对茴香胺和 2-氟丙二酸生产多公斤 3-氟-6-甲氧基喹啉的初步路线探索和最终工艺开发
由于需要多公斤量的这种杂环,因此需要开发一种可扩展的 3-氟-6-甲氧基喹啉路线。最初的路线开发侧重于通过 Balz-Schiemann 反应或使用 Selectfluor 的亲电氟化形成关键的 C-F 键。两种路线都是在实验室规模上开发的,并提供了克量的 3-fluoro-6-methoxyquinoline。然而,由于工艺安全问题和高步骤数,这两条路线都不适合进一步扩大规模。因此,开发了第三种方法,其中通过对茴香胺与 2-氟丙二酸缩合形成所需的杂环,这是两种廉价且可商购的原料。经过深入的优化和安全研究,这款 POCl 3介导的过程成功地扩大到 32 公斤的规模。最终加氢脱氯后,生产出 12 kg 纯度极佳的 3-fluoro-6-methoxyquinoline。
更新日期:2022-01-20
中文翻译:

从对茴香胺和 2-氟丙二酸生产多公斤 3-氟-6-甲氧基喹啉的初步路线探索和最终工艺开发
由于需要多公斤量的这种杂环,因此需要开发一种可扩展的 3-氟-6-甲氧基喹啉路线。最初的路线开发侧重于通过 Balz-Schiemann 反应或使用 Selectfluor 的亲电氟化形成关键的 C-F 键。两种路线都是在实验室规模上开发的,并提供了克量的 3-fluoro-6-methoxyquinoline。然而,由于工艺安全问题和高步骤数,这两条路线都不适合进一步扩大规模。因此,开发了第三种方法,其中通过对茴香胺与 2-氟丙二酸缩合形成所需的杂环,这是两种廉价且可商购的原料。经过深入的优化和安全研究,这款 POCl 3介导的过程成功地扩大到 32 公斤的规模。最终加氢脱氯后,生产出 12 kg 纯度极佳的 3-fluoro-6-methoxyquinoline。






























 京公网安备 11010802027423号
京公网安备 11010802027423号