当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of E6AP/UBE3A-Mediated Protein Ubiquitination and Degradation Pathways by a Cyclic γ-AA Peptide
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-01-19 , DOI: 10.1021/acs.jmedchem.1c01922 Bo Huang 1 , Li Zhou 2 , Ruochuan Liu 2 , Lei Wang 1 , Songyi Xue 1 , Yan Shi 1 , Geon Ho Jeong 2 , In Ho Jeong 2 , Sihao Li 1 , Jun Yin 2 , Jianfeng Cai 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-01-19 , DOI: 10.1021/acs.jmedchem.1c01922 Bo Huang 1 , Li Zhou 2 , Ruochuan Liu 2 , Lei Wang 1 , Songyi Xue 1 , Yan Shi 1 , Geon Ho Jeong 2 , In Ho Jeong 2 , Sihao Li 1 , Jun Yin 2 , Jianfeng Cai 1
Affiliation
|
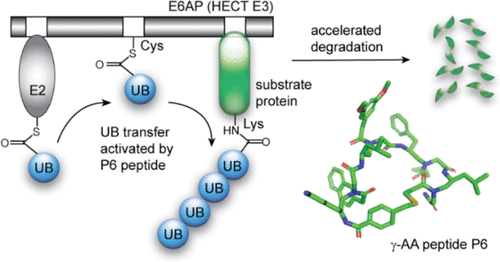
Manipulating the activities of E3 ubiquitin ligases with chemical ligands holds promise for correcting E3 malfunctions and repurposing the E3s for induced protein degradation in the cell. Herein, we report an alternative strategy to proteolysis-targeting chimeras (PROTACs) and molecular glues to induce protein degradation by constructing and screening a γ-AA peptide library for cyclic peptidomimetics binding to the HECT domain of E6AP, an E3 ubiquitinating p53 coerced by the human papillomavirus and regulating pathways implicated in neurodevelopmental disorders such as Angelman syndrome. We found that a γ-AA peptide P6, discovered from the affinity-based screening with the E6AP HECT domain, can significantly stimulate the ubiquitin ligase activity of E6AP to ubiquitinate its substrate proteins UbxD8, HHR23A, and β-catenin in reconstituted reactions and HEK293T cells. Furthermore, P6 can accelerate the degradation of E6AP substrates in the cell by enhancing the catalytic activities of E6AP. Our work demonstrates the feasibility of using synthetic ligands to stimulate E3 activities in the cell. The E3 stimulators could be developed alongside E3 inhibitors and substrate recruiters such as PROTACs and molecular glues to leverage the full potential of protein ubiquitination pathways for drug development.
中文翻译:

环状 γ-AA 肽激活 E6AP/UBE3A 介导的蛋白质泛素化和降解途径
用化学配体操纵 E3 泛素连接酶的活性有望纠正 E3 功能障碍,并重新利用 E3 来诱导细胞中的蛋白质降解。在此,我们报告了一种替代蛋白水解靶向嵌合体(PROTAC)和分子胶的策略,通过构建和筛选与 E6AP 的 HECT 结构域结合的 γ-AA 肽库来诱导蛋白质降解,E6AP 是一种由 E6AP 胁迫的 E3 泛素化 p53人乳头瘤病毒和与天使综合征等神经发育障碍有关的调节途径。我们发现,通过 E6AP HECT 结构域的亲和力筛选发现的 γ-AA 肽P6可以显着刺激 E6AP 的泛素连接酶活性,从而在重构反应和 HEK293T 中泛素化其底物蛋白 UbxD8、HHR23A 和 β-catenin细胞。此外, P6可以通过增强E6AP的催化活性来加速细胞内E6AP底物的降解。我们的工作证明了使用合成配体刺激细胞中 E3 活性的可行性。 E3 刺激剂可以与 E3 抑制剂和底物招募剂(例如 PROTAC 和分子胶)一起开发,以充分利用蛋白质泛素化途径的药物开发潜力。
更新日期:2022-02-10
中文翻译:

环状 γ-AA 肽激活 E6AP/UBE3A 介导的蛋白质泛素化和降解途径
用化学配体操纵 E3 泛素连接酶的活性有望纠正 E3 功能障碍,并重新利用 E3 来诱导细胞中的蛋白质降解。在此,我们报告了一种替代蛋白水解靶向嵌合体(PROTAC)和分子胶的策略,通过构建和筛选与 E6AP 的 HECT 结构域结合的 γ-AA 肽库来诱导蛋白质降解,E6AP 是一种由 E6AP 胁迫的 E3 泛素化 p53人乳头瘤病毒和与天使综合征等神经发育障碍有关的调节途径。我们发现,通过 E6AP HECT 结构域的亲和力筛选发现的 γ-AA 肽P6可以显着刺激 E6AP 的泛素连接酶活性,从而在重构反应和 HEK293T 中泛素化其底物蛋白 UbxD8、HHR23A 和 β-catenin细胞。此外, P6可以通过增强E6AP的催化活性来加速细胞内E6AP底物的降解。我们的工作证明了使用合成配体刺激细胞中 E3 活性的可行性。 E3 刺激剂可以与 E3 抑制剂和底物招募剂(例如 PROTAC 和分子胶)一起开发,以充分利用蛋白质泛素化途径的药物开发潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号