当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study of 1,2-Dichloroethane Hydrodechlorination on Cu-Rich Pt–Cu Alloys: Combining Reaction Kinetics Experiments with DFT Calculations and Microkinetic Modeling
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-01-19 , DOI: 10.1021/acssuschemeng.1c06899 Lang Xu 1 , Eric Stangland 2 , James A. Dumesic 1 , Manos Mavrikakis 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-01-19 , DOI: 10.1021/acssuschemeng.1c06899 Lang Xu 1 , Eric Stangland 2 , James A. Dumesic 1 , Manos Mavrikakis 1
Affiliation
|
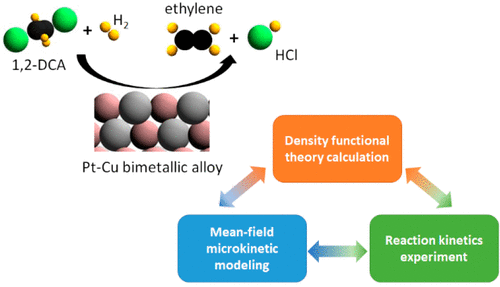
Cu-rich Pt–Cu bimetallic catalysts are among the most promising candidates for actively catalyzing the hydrodechlorination of 1,2-dichloroethane (1,2-DCA) toward ethylene production. Combining reaction kinetics experiments with density functional theory (DFT) calculations and mean-field microkinetic modeling, we present a systematic mechanistic study for 1,2-DCA hydrodechlorination on Cu-rich Pt–Cu alloy catalysts. Our DFT (PBE+(TS+SCS)) results suggest that increasing Cu content in the Pt–Cu alloy destabilizes C2-species adsorption while stabilizing the binding of atomic chlorine. The reaction energetics of all the elementary steps in the 1,2-DCA reaction network were calculated on a Pt1Cu3(111) model surface. The DFT results were then used to construct a microkinetic model, and the model-predicted reaction rates were compared with our reaction kinetics experimental results on a Cu-rich SiO2-supported Pt–Cu alloy catalyst through a parameter estimation procedure. Both the reaction kinetics experiments and the microkinetic model after parameter adjustments yielded 100% selectivity to ethylene. The microkinetic model pointed to a reaction pathway involving two sequential chlorine-removal steps on the Pt–Cu alloy catalyst, a mechanism distinct from the one previously identified on pure Pt/SiO2 catalysts, which involved an initial hydrogen-removal step. Adjustments to the DFT-derived parameters indicate the possible formation of chlorine-induced Cu-enriched surface sites during 1,2-DCA hydrodechlorination conditions, sites that are more active than those encountered in the bulk Pt1Cu3(111) alloy surface. Our study offers valuable initial insights on the 1,2-DCA hydrodechlorination reaction mechanism and the nature of the active sites on PtCu bimetallic catalysts.
中文翻译:

富铜 Pt-Cu 合金上 1,2-二氯乙烷加氢脱氯的机理研究:将反应动力学实验与 DFT 计算和微动力学建模相结合
富铜 Pt-Cu 双金属催化剂是活性催化 1,2-二氯乙烷 (1,2-DCA) 加氢脱氯制乙烯的最有希望的候选者之一。我们将反应动力学实验与密度泛函理论 (DFT) 计算和平均场微动力学建模相结合,对富铜 Pt-Cu 合金催化剂上的 1,2-DCA 加氢脱氯进行了系统的机理研究。我们的 DFT (PBE+(TS+SCS)) 结果表明,增加 Pt-Cu 合金中的 Cu 含量会破坏 C 2物质的吸附,同时稳定原子氯的结合。在 Pt 1 Cu 3上计算了 1,2-DCA 反应网络中所有基本步骤的反应能量学(111) 模型表面。然后将 DFT 结果用于构建微动力学模型,并通过参数估计程序将模型预测的反应速率与我们在富铜 SiO 2负载的 Pt-Cu 合金催化剂上的反应动力学实验结果进行比较。参数调整后的反应动力学实验和微动力学模型均产生了 100% 的乙烯选择性。微动力学模型指出了一种反应途径,涉及 Pt-Cu 合金催化剂上的两个连续除氯步骤,该机理不同于先前在纯 Pt/SiO 2上确定的机理催化剂,其中涉及初始的除氢步骤。对 DFT 衍生参数的调整表明,在 1,2-DCA 加氢脱氯条件下,可能会形成氯诱导的富铜表面位点,这些位点比在块状 Pt 1 Cu 3 (111) 合金表面中遇到的位点更活跃。我们的研究为 1,2-DCA 加氢脱氯反应机理和 PtCu 双金属催化剂上活性位点的性质提供了有价值的初步见解。
更新日期:2022-01-31
中文翻译:

富铜 Pt-Cu 合金上 1,2-二氯乙烷加氢脱氯的机理研究:将反应动力学实验与 DFT 计算和微动力学建模相结合
富铜 Pt-Cu 双金属催化剂是活性催化 1,2-二氯乙烷 (1,2-DCA) 加氢脱氯制乙烯的最有希望的候选者之一。我们将反应动力学实验与密度泛函理论 (DFT) 计算和平均场微动力学建模相结合,对富铜 Pt-Cu 合金催化剂上的 1,2-DCA 加氢脱氯进行了系统的机理研究。我们的 DFT (PBE+(TS+SCS)) 结果表明,增加 Pt-Cu 合金中的 Cu 含量会破坏 C 2物质的吸附,同时稳定原子氯的结合。在 Pt 1 Cu 3上计算了 1,2-DCA 反应网络中所有基本步骤的反应能量学(111) 模型表面。然后将 DFT 结果用于构建微动力学模型,并通过参数估计程序将模型预测的反应速率与我们在富铜 SiO 2负载的 Pt-Cu 合金催化剂上的反应动力学实验结果进行比较。参数调整后的反应动力学实验和微动力学模型均产生了 100% 的乙烯选择性。微动力学模型指出了一种反应途径,涉及 Pt-Cu 合金催化剂上的两个连续除氯步骤,该机理不同于先前在纯 Pt/SiO 2上确定的机理催化剂,其中涉及初始的除氢步骤。对 DFT 衍生参数的调整表明,在 1,2-DCA 加氢脱氯条件下,可能会形成氯诱导的富铜表面位点,这些位点比在块状 Pt 1 Cu 3 (111) 合金表面中遇到的位点更活跃。我们的研究为 1,2-DCA 加氢脱氯反应机理和 PtCu 双金属催化剂上活性位点的性质提供了有价值的初步见解。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号