当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NHC-Catalyzed 1,4-Elimination in the Dearomative Activation of 3-Furaldehydes towards (4+2)-Cycloadditions
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-01-18 , DOI: 10.1002/adsc.202101338 Artur Przydacz 1 , Aleksandra Topolska 1 , Anna Skrzyńska 1 , Łukasz Albrecht 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-01-18 , DOI: 10.1002/adsc.202101338 Artur Przydacz 1 , Aleksandra Topolska 1 , Anna Skrzyńska 1 , Łukasz Albrecht 1
Affiliation
|
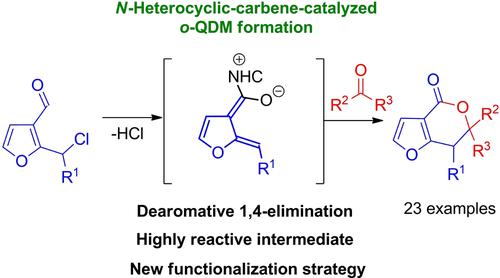
A dearomative formal (4+2)-cycloaddition reaction between 2-substituted 3-furaldehydes derivatives and isatins or α,α,α-trifluoroacetophenones as electrophiles has been established under NHC-catalysis. This approach utilizes the process of hydrogen chloride 1,4-elimination leading to a highly reactive NHC-bound heterocyclic o-QDM intermediates derived from 3-furaldehydes, which play a key role in the process. By using this strategy, a series of structurally diverse 6,7-dihydro-4H-furo[3,2-c]pyran-4-ones was prepared in 41–85% yields. In addition, the potential of the obtained (4+2)-cycloadducts has been confirmed in the synthesis of interesting class of spirooxindole derivative containing 6,7-dihydro-4H-furo[3,2-c]pyran scaffold.
中文翻译:

NHC 催化 1,4-消除在 3-糠醛对 (4+2)-环加成反应中的脱芳烃活化
已在 NHC 催化下建立了 2-取代的 3-糠醛衍生物与靛红或 α,α,α-三氟苯乙酮之间的脱芳构 (4+2)-环加成反应。该方法利用氯化氢 1,4-消除过程,产生高反应性的 NHC 结合的杂环o - QDM 中间体,该中间体衍生自 3-糠醛,在该过程中起关键作用。通过使用该策略,一系列结构多样的 6,7-dihydro-4 H -furo [3,2 - c ]pyran-4-ones 以 41-85% 的产率制备。此外,所获得的 (4+2)-环加合物的潜力已在合成含有 6,7-dihydro-4 H -furo[3,2- c的有趣类螺吲哚衍生物中得到证实。]吡喃支架。
更新日期:2022-01-18
中文翻译:

NHC 催化 1,4-消除在 3-糠醛对 (4+2)-环加成反应中的脱芳烃活化
已在 NHC 催化下建立了 2-取代的 3-糠醛衍生物与靛红或 α,α,α-三氟苯乙酮之间的脱芳构 (4+2)-环加成反应。该方法利用氯化氢 1,4-消除过程,产生高反应性的 NHC 结合的杂环o - QDM 中间体,该中间体衍生自 3-糠醛,在该过程中起关键作用。通过使用该策略,一系列结构多样的 6,7-dihydro-4 H -furo [3,2 - c ]pyran-4-ones 以 41-85% 的产率制备。此外,所获得的 (4+2)-环加合物的潜力已在合成含有 6,7-dihydro-4 H -furo[3,2- c的有趣类螺吲哚衍生物中得到证实。]吡喃支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号