当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
RhIII-Catalyzed heteroarylation of N-2,6-difluorophenyl arylamides with heteroaryl boronate esters
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-12-29 , DOI: 10.1039/d1qo01868j Jia-Xue Wu 1 , Qing-Xia Yao 1 , Wen-Zeng Duan 1 , Da-Cheng Li 1 , Xian-Qiang Huang 1 , Jian-Min Dou 1 , Huai-Wei Wang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-12-29 , DOI: 10.1039/d1qo01868j Jia-Xue Wu 1 , Qing-Xia Yao 1 , Wen-Zeng Duan 1 , Da-Cheng Li 1 , Xian-Qiang Huang 1 , Jian-Min Dou 1 , Huai-Wei Wang 1
Affiliation
|
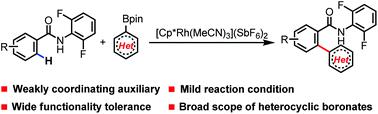
Herein, an efficient strategy to achieve aryl-heteroaryl formation via RhIII-catalyzed ortho-C(sp2)–H heteroarylation of (hetero)arenes with heterocyclic boronates using a readily removable N-2,6-difluorophenyl arylamide directing group has been disclosed. A variety of heteroaromatic boronates as the coupling partners were shown to be able to participate in this protocol, providing the desired heteroarylated products with high reactivity and good tolerance of functional groups. The achievement of this C(sp2)–H heteroarylation could potentially offer a route to synthesize heterocyclic drug molecules in medicinal chemistry.
中文翻译:

RhIII-催化 N-2,6-二氟苯基芳基酰胺与杂芳基硼酸酯的杂芳基化反应
在此,通过Rh III催化的(杂)芳烃与杂环硼酸酯的邻-C(sp 2 )-H 杂芳基化,使用易于去除的N -2,6-二氟苯基芳基酰胺导向基团来实现芳基-杂芳基形成的有效策略是披露。多种杂芳硼酸盐作为偶联伙伴被证明能够参与该协议,提供具有高反应性和良好的官能团耐受性的所需杂芳基化产品。这种 C(sp 2 )-H 杂芳基化的实现可能为在药物化学中合成杂环药物分子提供了一条途径。
更新日期:2022-01-19
中文翻译:

RhIII-催化 N-2,6-二氟苯基芳基酰胺与杂芳基硼酸酯的杂芳基化反应
在此,通过Rh III催化的(杂)芳烃与杂环硼酸酯的邻-C(sp 2 )-H 杂芳基化,使用易于去除的N -2,6-二氟苯基芳基酰胺导向基团来实现芳基-杂芳基形成的有效策略是披露。多种杂芳硼酸盐作为偶联伙伴被证明能够参与该协议,提供具有高反应性和良好的官能团耐受性的所需杂芳基化产品。这种 C(sp 2 )-H 杂芳基化的实现可能为在药物化学中合成杂环药物分子提供了一条途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号