当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complex Hydrides with (BH4)−and (NH2)−Anions as New Lithium Fast-Ion Conductors
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-11-18 , DOI: 10.1021/ja907249p
Motoaki Matsuo 1 , Arndt Remhof 1 , Pascal Martelli 1 , Riccarda Caputo 1 , Matthias Ernst 1 , Yohei Miura 1 , Toyoto Sato 1 , Hiroyuki Oguchi 1 , Hideki Maekawa 1 , Hitoshi Takamura 1 , Andreas Borgschulte 1 , Andreas Züttel 1 , Shin-ichi Orimo 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-11-18 , DOI: 10.1021/ja907249p
Motoaki Matsuo 1 , Arndt Remhof 1 , Pascal Martelli 1 , Riccarda Caputo 1 , Matthias Ernst 1 , Yohei Miura 1 , Toyoto Sato 1 , Hiroyuki Oguchi 1 , Hideki Maekawa 1 , Hitoshi Takamura 1 , Andreas Borgschulte 1 , Andreas Züttel 1 , Shin-ichi Orimo 1
Affiliation
|
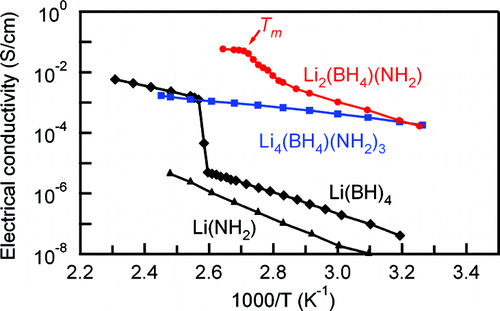
Some of the authors have reported that a complex hydride, Li(BH(4)), with the (BH(4))(-) anion exhibits lithium fast-ion conduction (more than 1 x 10(-3) S/cm) accompanied by the structural transition at approximately 390 K for the first time in 30 years since the conduction in Li(2)(NH) was reported in 1979. Here we report another conceptual study and remarkable results of Li(2)(BH(4))(NH(2)) and Li(4)(BH(4))(NH(2))(3) combined with the (BH(4))(-) and (NH(2))(-) anions showing ion conductivities 4 orders of magnitude higher than that for Li(BH(4)) at RT, due to being provided with new occupation sites for Li(+) ions. Both Li(2)(BH(4))(NH(2)) and Li(4)(BH(4))(NH(2))(3) exhibit a lithium fast-ion conductivity of 2 x 10(-4) S/cm at RT, and the activation energy for conduction in Li(4)(BH(4))(NH(2))(3) is evaluated to be 0.26 eV, less than half those in Li(2)(BH(4))(NH(2)) and Li(BH(4)). This study not only demonstrates an important direction in which to search for higher ion conductivity in complex hydrides but also greatly increases the material variations of solid electrolytes.
中文翻译:

具有 (BH4)-和 (NH2)-阴离子的复合氢化物作为新型锂快离子导体
一些作者报告说,具有 (BH(4))(-) 阴离子的复合氢化物 Li(BH(4)) 表现出锂快离子传导(超过 1 x 10(-3) S/cm ) 伴随着自 1979 年报道 Li(2)(NH) 的传导以来 30 年来首次在大约 390 K 处发生结构转变。在这里我们报告了 Li(2)(BH( 4))(NH(2)) 和 Li(4)(BH(4))(NH(2))(3) 与 (BH(4))(-) 和 (NH(2))(- ) 阴离子显示离子电导率比 Li(BH(4)) 在 RT 高 4 个数量级,这是由于为 Li(+) 离子提供了新的占据位点。Li(2)(BH(4))(NH(2)) 和 Li(4)(BH(4))(NH(2))(3) 均表现出 2 x 10(- 4) 室温下的 S/cm,Li(4)(BH(4))(NH(2))(3) 中的传导活化能估计为 0.26 eV,不到 Li(2)(BH(4))(NH(2)) 和 Li(BH(4)) 中的一半。该研究不仅为在复合氢化物中寻找更高离子电导率提供了一个重要方向,而且大大增加了固体电解质的材料变化。
更新日期:2009-11-18
中文翻译:

具有 (BH4)-和 (NH2)-阴离子的复合氢化物作为新型锂快离子导体
一些作者报告说,具有 (BH(4))(-) 阴离子的复合氢化物 Li(BH(4)) 表现出锂快离子传导(超过 1 x 10(-3) S/cm ) 伴随着自 1979 年报道 Li(2)(NH) 的传导以来 30 年来首次在大约 390 K 处发生结构转变。在这里我们报告了 Li(2)(BH( 4))(NH(2)) 和 Li(4)(BH(4))(NH(2))(3) 与 (BH(4))(-) 和 (NH(2))(- ) 阴离子显示离子电导率比 Li(BH(4)) 在 RT 高 4 个数量级,这是由于为 Li(+) 离子提供了新的占据位点。Li(2)(BH(4))(NH(2)) 和 Li(4)(BH(4))(NH(2))(3) 均表现出 2 x 10(- 4) 室温下的 S/cm,Li(4)(BH(4))(NH(2))(3) 中的传导活化能估计为 0.26 eV,不到 Li(2)(BH(4))(NH(2)) 和 Li(BH(4)) 中的一半。该研究不仅为在复合氢化物中寻找更高离子电导率提供了一个重要方向,而且大大增加了固体电解质的材料变化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号