当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of the SgcF Epoxide Hydrolase Supporting an (R)-Vicinal Diol Intermediate for Enediyne Antitumor Antibiotic C-1027 Biosynthesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-11-18 , DOI: 10.1021/ja901242s Shuangjun Lin 1 , Geoffrey P Horsman , Yihua Chen , Wenli Li , Ben Shen
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-11-18 , DOI: 10.1021/ja901242s Shuangjun Lin 1 , Geoffrey P Horsman , Yihua Chen , Wenli Li , Ben Shen
Affiliation
|
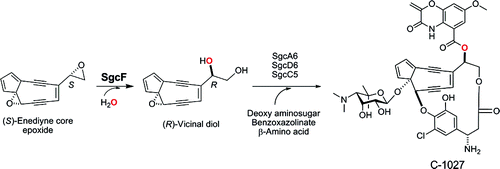
C-1027 is a chromoprotein antitumor antibiotic consisting of an apoprotein and the C-1027 chromophore. The C-1027 chromophore possesses four distinct structural moieties-an enediyne core, a deoxy aminosugar, a benzoxazolinate, and an (S)-3-chloro-5-hydroxy-beta-tyrosine-the latter two of which are proposed to be appended to the enediyne core via a convergent biosynthetic strategy. Here we report the in vitro characterization of SgcF, an epoxide hydrolase from the C-1027 biosynthetic gene cluster that catalyzes regio- and stereospecific hydrolysis of styrene oxide, serving as an enediyne core epoxide intermediate mimic, to form a vicinal diol. Abolishment of C-1027 production in the DeltasgcF mutant strain Streptomyces globisporus SB1010 unambiguously establishes that sgcF plays an indispensable role in C-1027 biosynthesis. SgcF efficiently hydrolyzes (S)-styrene oxide, displaying an apparent K(m) of 0.6 +/- 0.1 mM and k(cat) of 48 +/- 1 min(-1), via attack at the alpha-position to exclusively generate the (R)-phenyl vicinal diol, consistent with the stereochemistry of the C-1027 chromophore. These findings support the role of SgcF in the proposed convergent pathway for C-1027 biosynthesis, unveiling an (R)-vicinal diol as a key intermediate. Interestingly, SgcF can also hydrolyze (R)-styrene oxide to afford preferentially the (R)-phenyl vicinal diol via attack at the beta-position, albeit with significantly reduced efficiency (apparent K(m) of 2.0 +/- 0.4 mM and k(cat) = 4.3 +/- 0.3 min(-1)). Although the latter activity unlikely contributes to C-1027 biosynthesis in vivo, such enantioconvergence arising from complementary regioselective hydrolysis of a racemic substrate could be exploited to engineer epoxide hydrolases with improved regio- and/or enantiospecificity.
中文翻译:

支持用于烯二炔抗肿瘤抗生素 C-1027 生物合成的 (R)-邻二醇中间体的 SgcF 环氧化物水解酶的表征
C-1027 是一种色蛋白抗肿瘤抗生素,由载脂蛋白和 C-1027 生色团组成。C-1027 发色团具有四个不同的结构部分——一个烯二炔核心、一个脱氧氨基糖、一个苯并恶唑啉酸盐和一个 (S)-3-氯-5-羟基-β-酪氨酸——后两个被提议附加通过收敛的生物合成策略到达烯二炔核心。在这里,我们报告了 SgcF 的体外表征,这是一种来自 C-1027 生物合成基因簇的环氧化物水解酶,可催化氧化苯乙烯的区域和立体定向水解,作为烯二炔核心环氧化物中间体模拟物,形成邻二醇。DeltasgcF 突变菌株 Streptomyces globisporus SB1010 中 C-1027 生产的废除明确表明 sgcF 在 C-1027 生物合成中起着不可或缺的作用。SgcF 有效地水解 (S)-氧化苯乙烯,显示表观 K(m) 为 0.6 +/- 0.1 mM 和 k(cat) 为 48 +/- 1 min(-1),通过在 alpha 位置进行攻击以专门生成 (R)-苯基邻二醇,与 C-1027 发色团的立体化学一致。这些发现支持 SgcF 在拟议的 C-1027 生物合成收敛途径中的作用,揭示了 (R)-邻二醇作为关键中间体。有趣的是,SgcF 还可以水解 (R)-氧化苯乙烯,通过攻击 β 位优先提供 (R)-苯基邻二醇,尽管效率显着降低(表观 K(m) 为 2.0 +/- 0.4 mM 和k(猫)= 4.3 +/- 0.3 分钟(-1))。尽管后一种活性不太可能有助于体内 C-1027 的生物合成,
更新日期:2009-11-18
中文翻译:

支持用于烯二炔抗肿瘤抗生素 C-1027 生物合成的 (R)-邻二醇中间体的 SgcF 环氧化物水解酶的表征
C-1027 是一种色蛋白抗肿瘤抗生素,由载脂蛋白和 C-1027 生色团组成。C-1027 发色团具有四个不同的结构部分——一个烯二炔核心、一个脱氧氨基糖、一个苯并恶唑啉酸盐和一个 (S)-3-氯-5-羟基-β-酪氨酸——后两个被提议附加通过收敛的生物合成策略到达烯二炔核心。在这里,我们报告了 SgcF 的体外表征,这是一种来自 C-1027 生物合成基因簇的环氧化物水解酶,可催化氧化苯乙烯的区域和立体定向水解,作为烯二炔核心环氧化物中间体模拟物,形成邻二醇。DeltasgcF 突变菌株 Streptomyces globisporus SB1010 中 C-1027 生产的废除明确表明 sgcF 在 C-1027 生物合成中起着不可或缺的作用。SgcF 有效地水解 (S)-氧化苯乙烯,显示表观 K(m) 为 0.6 +/- 0.1 mM 和 k(cat) 为 48 +/- 1 min(-1),通过在 alpha 位置进行攻击以专门生成 (R)-苯基邻二醇,与 C-1027 发色团的立体化学一致。这些发现支持 SgcF 在拟议的 C-1027 生物合成收敛途径中的作用,揭示了 (R)-邻二醇作为关键中间体。有趣的是,SgcF 还可以水解 (R)-氧化苯乙烯,通过攻击 β 位优先提供 (R)-苯基邻二醇,尽管效率显着降低(表观 K(m) 为 2.0 +/- 0.4 mM 和k(猫)= 4.3 +/- 0.3 分钟(-1))。尽管后一种活性不太可能有助于体内 C-1027 的生物合成,































 京公网安备 11010802027423号
京公网安备 11010802027423号