Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2022-01-17 , DOI: 10.1016/j.bioorg.2021.105592 Mehdi Asadi 1 , Aida Iraji 2 , Maede Sherafati 3 , Mohammad Nazari Montazer 3 , Shirin Ansari 4 , Maryam Mohammadi Khanaposhtani 5 , Nader Tanideh 6 , Mehdi Dianatpour 6 , Mahmood Biglar 7 , Bagher Larijani 7 , Alireza Foroumadi 3 , Homa Azizian 7 , Massoud Amanlou 3 , Mohammad Mahdavi 7
|
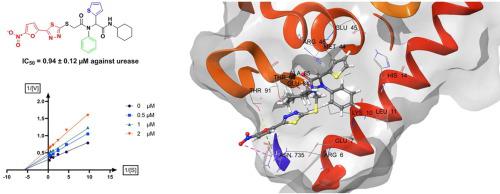
A series of 5-nitrofuran-2-yl-thiadiazole linked to different cyclohexyl-2-(phenylamino)acetamides were rationally designed and synthesized. All synthetic compounds were evaluated for their urease inhibitory activity and exhibited good inhibitory potential against urease with IC50 values in the range of 0.94 – 6.78 μM as compared to the standard thiourea (IC50 = 22.50 μM). Compound 8g (IC50 = 0.94 μM) with a thiophene substituent at the R2 position was found to be the most active member of the series. Kinetic studies exhibited that the compound 8g was a non-competitive inhibitor. In silico study showed the critical interactions of potent inhibitors with the active site of the enzyme. These newly identified inhibitors of the urease enzyme can serve as leads for further research and development.
中文翻译:

5-nitrofuran-2-yl-thiadiazole 与不同 cyclohexyl-2-(phenylamino)acetamides 的合成及体外脲酶抑制活性,计算机和动力学研究
合理设计合成了一系列与不同环己基-2-(苯基氨基)乙酰胺相连的5-硝基呋喃-2-基-噻二唑。评估了所有合成化合物的脲酶抑制活性,并显示出对脲酶的良好抑制潜力,与标准硫脲 (IC 50 = 22.50 μM)相比, IC 50值在 0.94 – 6.78 μM 范围内。发现在 R 2位 具有噻吩取代基的化合物8g (IC 50 = 0.94 μM)是该系列中最活跃的成员。动力学研究表明化合物8g是一种非竞争性抑制剂。计算机 研究表明,强效抑制剂与酶的活性位点之间存在关键的相互作用。这些新发现的脲酶抑制剂可以作为进一步研究和开发的线索。

































 京公网安备 11010802027423号
京公网安备 11010802027423号