当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and mechanism of Thallium(I) oxidation by Permanganate: Role of bromide
Chemosphere ( IF 8.1 ) Pub Date : 2022-01-17 , DOI: 10.1016/j.chemosphere.2022.133652 Chengxue Ma 1 , Xiaoliu Huangfu 2 , Yijie Zou 2 , Ruixing Huang 1 , Qiang He 2 , Jun Ma 1
Chemosphere ( IF 8.1 ) Pub Date : 2022-01-17 , DOI: 10.1016/j.chemosphere.2022.133652 Chengxue Ma 1 , Xiaoliu Huangfu 2 , Yijie Zou 2 , Ruixing Huang 1 , Qiang He 2 , Jun Ma 1
Affiliation
|
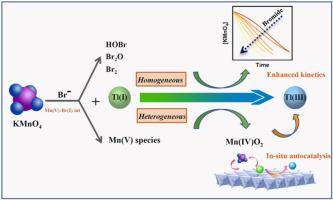
The oxidation of thallium(I) (Tl (I)) to Tl (III) is referred to as an efficient means for Tl removal. Bromide (Br‾) inevitably occurs in nearly all water sources at concentrations of 0.01–67 mg/L (0.14–960 μM). The effect of Br‾ remains largely unclear but likely of critical importance on the redox fate and thus the removal potential of Tl (I) during typical oxidation treatment processes. Here, we investigate the kinetics and tackle the mechanism of Tl (I) oxidation by permanganate (KMnO4 ) under the influence of Br‾. The results demonstrated that Br‾ at environmental levels exhibited significant catalytic effect on Tl (I) oxidation kinetics by KMnO4 at acidic pH of 4.0–7.0, while no significant effect of Br‾ was observed for Tl(I) oxidation under alkaline conditions of pH 8.0 and 9.0. It was found that the enhanced oxidation kinetics under acidic conditions was driven by the combined effect of and autocatalysis mediated by MnO2 and a fast oxidation kinetics served by in-situ formed bromine species. Through quantifying the relative contributions of those bromine species to the homogenous oxidation of Tl(I), HOBr, Br2 and Br2 O were found to play roles in catalyzing the oxidation of Tl(I) by KMnO4 . The results discussed herein highlight the critical role of Br‾ on the Tl(I) complex oxidation process by KMnO4 and may have implications for evaluating the redox cycle and removal potential of Tl in bromide-containing water treatment.
中文翻译:

高锰酸盐氧化铊 (I) 的动力学和机理:溴化物的作用
铊 (I) (Tl (I)) 氧化成 Tl (III) 被称为去除 Tl 的有效方法。溴化物 (Br ̅) 不可避免地存在于几乎所有水源中,浓度为 0.01–67 mg/L (0.14–960 μM)。Br ̅ 的影响在很大程度上仍不清楚,但可能对氧化还原命运至关重要,从而在典型的氧化处理过程中对 Tl (I) 的去除潜力至关重要。在这里,我们研究了动力学并解决了在 Br ̅ 影响下高锰酸盐 (KMnO4) 氧化 Tl (I) 的机制。结果表明,在 pH 值为 4.0–7.0 的酸性 pH 条件下,环境水平的 Br ̅ 对 KMnO4 的 Tl (I) 氧化动力学表现出显著的催化作用,而在 pH 值为 8.0 和 9.0 的碱性条件下,Br ̅ 对 Tl(I) 氧化没有显着影响。研究发现,酸性条件下增强的氧化动力学是由 MnO2 介导的自催化和原位形成的溴物质提供的快速氧化动力学的联合作用驱动的。通过量化这些溴物质对 Tl(I) 均匀氧化的相对贡献,发现 HOBr、Br2 和 Br2O 在催化 KMnO 4 氧化 Tl(I) 中发挥作用。本文讨论的结果强调了 Br ̅ 在 KMnO4 的 Tl(I) 络合物氧化过程中的关键作用,可能对评估含溴化物水处理中 Tl 的氧化还原循环和去除潜力具有意义。
更新日期:2022-01-17
中文翻译:

高锰酸盐氧化铊 (I) 的动力学和机理:溴化物的作用
铊 (I) (Tl (I)) 氧化成 Tl (III) 被称为去除 Tl 的有效方法。溴化物 (Br ̅) 不可避免地存在于几乎所有水源中,浓度为 0.01–67 mg/L (0.14–960 μM)。Br ̅ 的影响在很大程度上仍不清楚,但可能对氧化还原命运至关重要,从而在典型的氧化处理过程中对 Tl (I) 的去除潜力至关重要。在这里,我们研究了动力学并解决了在 Br ̅ 影响下高锰酸盐 (KMnO4) 氧化 Tl (I) 的机制。结果表明,在 pH 值为 4.0–7.0 的酸性 pH 条件下,环境水平的 Br ̅ 对 KMnO4 的 Tl (I) 氧化动力学表现出显著的催化作用,而在 pH 值为 8.0 和 9.0 的碱性条件下,Br ̅ 对 Tl(I) 氧化没有显着影响。研究发现,酸性条件下增强的氧化动力学是由 MnO2 介导的自催化和原位形成的溴物质提供的快速氧化动力学的联合作用驱动的。通过量化这些溴物质对 Tl(I) 均匀氧化的相对贡献,发现 HOBr、Br2 和 Br2O 在催化 KMnO 4 氧化 Tl(I) 中发挥作用。本文讨论的结果强调了 Br ̅ 在 KMnO4 的 Tl(I) 络合物氧化过程中的关键作用,可能对评估含溴化物水处理中 Tl 的氧化还原循环和去除潜力具有意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号