当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct conversion of CO2 to solid carbon by Ga-based liquid metals
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-01-17 , DOI: 10.1039/d1ee03283f
Karma Zuraiqi 1 , Ali Zavabeti 1, 2 , Jonathan Clarke-Hannaford 3 , Billy James Murdoch 3 , Kalpit Shah 1 , Michelle J. S. Spencer 3 , Chris F. McConville 4 , Torben Daeneke 1 , Ken Chiang 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2022-01-17 , DOI: 10.1039/d1ee03283f
Karma Zuraiqi 1 , Ali Zavabeti 1, 2 , Jonathan Clarke-Hannaford 3 , Billy James Murdoch 3 , Kalpit Shah 1 , Michelle J. S. Spencer 3 , Chris F. McConville 4 , Torben Daeneke 1 , Ken Chiang 1
Affiliation
|
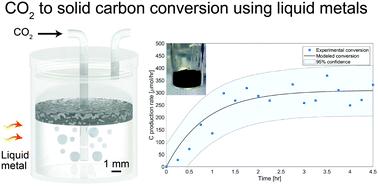
The direct conversion of CO2 to carbon is a highly providential route; however, conventional thermal and catalytic approaches are hindered by high energy demands and are limited by coking. Here, we report a robust and highly selective method for the direct conversion of CO2 to solid carbon over EGaIn liquid metal (LM) alloy. We utilized the low-melting point of this alloy to facilitate the reduction of CO2 at low temperatures, producing 319 μmol h−1 of carbon at 200 °C, and remarkably enabling CO2 activation and carbon production even at room temperature, without the use of a supplementary reductant such as hydrogen. The deployed LM showed no signs of deactivation by coking and the generated carbon is shown to naturally accumulate at the top of the LM where it can be easily collected. In situ XPS measurements indicate an increase of 9.6% in the carbon–carbon bond content and an equivalent decrease in the Ga metal content, upon exposure of the LM to CO2 for 30 mins at 200 °C and 1 bar. This led to the conclusion that solid carbon and gallium oxide are the final reaction products of this process. Density functional theory calculations shed further light on the adsorption and dissociation of CO2 over Ga and EGaIn. The presented method creates a pathway to transforming CO2 to perpetually stored solid carbon and can therefore set a trajectory for making a measurable impact on carbon intensive industries.
中文翻译:

Ga基液态金属将CO2直接转化为固体碳
CO 2直接转化为碳是一条非常自然的路线。然而,传统的热和催化方法受到高能量需求的阻碍,并受到焦化的限制。在这里,我们报告了一种稳健且高选择性的方法,用于通过 EGaIn 液态金属 (LM) 合金将 CO 2直接转化为固体碳。我们利用这种合金的低熔点来促进低温下CO 2的还原,在 200 °C 时产生 319 μmol h -1的碳,并显着使 CO 2即使在室温下也能进行活化和碳生成,而无需使用氢气等辅助还原剂。部署的 LM 没有显示因焦化而失活的迹象,生成的碳显示在 LM 的顶部自然积聚,可以很容易地收集。原位XPS 测量表明,在 200 °C 和 1 bar下将 LM 暴露于 CO 2 30 分钟后,碳-碳键含量增加了 9.6%,而 Ga 金属含量则相应减少。由此得出结论,固体碳和氧化镓是该过程的最终反应产物。密度泛函理论计算进一步阐明了 CO 2的吸附和解离在 Ga 和 EGaIn 上。所提出的方法创造了将 CO 2转化为永久储存的固体碳的途径,因此可以为对碳密集型产业产生可衡量的影响设定轨迹。
更新日期:2022-01-17
中文翻译:

Ga基液态金属将CO2直接转化为固体碳
CO 2直接转化为碳是一条非常自然的路线。然而,传统的热和催化方法受到高能量需求的阻碍,并受到焦化的限制。在这里,我们报告了一种稳健且高选择性的方法,用于通过 EGaIn 液态金属 (LM) 合金将 CO 2直接转化为固体碳。我们利用这种合金的低熔点来促进低温下CO 2的还原,在 200 °C 时产生 319 μmol h -1的碳,并显着使 CO 2即使在室温下也能进行活化和碳生成,而无需使用氢气等辅助还原剂。部署的 LM 没有显示因焦化而失活的迹象,生成的碳显示在 LM 的顶部自然积聚,可以很容易地收集。原位XPS 测量表明,在 200 °C 和 1 bar下将 LM 暴露于 CO 2 30 分钟后,碳-碳键含量增加了 9.6%,而 Ga 金属含量则相应减少。由此得出结论,固体碳和氧化镓是该过程的最终反应产物。密度泛函理论计算进一步阐明了 CO 2的吸附和解离在 Ga 和 EGaIn 上。所提出的方法创造了将 CO 2转化为永久储存的固体碳的途径,因此可以为对碳密集型产业产生可衡量的影响设定轨迹。

































 京公网安备 11010802027423号
京公网安备 11010802027423号