当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (Thio)Furan-Fused Phospholes via Phosphonation Cyclization and a Base-Promoted Phospha-Friedel–Crafts Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.joc.1c02577 Chenchen Li 1 , Haiyang Huang 1 , Fen Liu 1 , Chengxiong Yuan 1 , Siyu Chen 1 , Yuhui Hua 1 , Haixin Ding 1 , Xian-Rong Song 1 , Qiang Xiao 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-01-14 , DOI: 10.1021/acs.joc.1c02577 Chenchen Li 1 , Haiyang Huang 1 , Fen Liu 1 , Chengxiong Yuan 1 , Siyu Chen 1 , Yuhui Hua 1 , Haixin Ding 1 , Xian-Rong Song 1 , Qiang Xiao 1
Affiliation
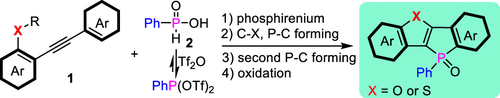
|
Herein, we developed a novel strategy for synthesizing ladder (thio)furan-fused phospholes via intermolecular phosphonation cyclization and a base-promoted phospha-Friedel–Crafts reaction under mild conditions. The starting substrates are readily available phosphinic acids and easy-to-handle alkynes. The details of the reaction mechanism were further rationalized using theoretical calculations. This protocol can be widely applied to synthesize furan- and thiofuran-fused phospholes as well as the corresponding large π-extended derivatives, which are of great interest in the domain of organic functional materials.
中文翻译:

通过磷化环化和碱基促进的磷酸-弗里德尔-克拉夫茨反应合成(硫代)呋喃稠合磷酸
在此,我们开发了一种在温和条件下通过分子间磷酸化环化和碱基促进的磷酸-弗里德尔-克拉夫茨反应合成梯状(硫代)呋喃稠合磷酸酯的新策略。起始底物是容易获得的次膦酸和易于处理的炔烃。使用理论计算进一步合理化了反应机理的细节。该协议可广泛应用于合成呋喃和硫代呋喃稠合磷酸酯以及相应的大 π 扩展衍生物,这些衍生物在有机功能材料领域具有重要意义。
更新日期:2022-01-14
中文翻译:

通过磷化环化和碱基促进的磷酸-弗里德尔-克拉夫茨反应合成(硫代)呋喃稠合磷酸
在此,我们开发了一种在温和条件下通过分子间磷酸化环化和碱基促进的磷酸-弗里德尔-克拉夫茨反应合成梯状(硫代)呋喃稠合磷酸酯的新策略。起始底物是容易获得的次膦酸和易于处理的炔烃。使用理论计算进一步合理化了反应机理的细节。该协议可广泛应用于合成呋喃和硫代呋喃稠合磷酸酯以及相应的大 π 扩展衍生物,这些衍生物在有机功能材料领域具有重要意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号