Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface-Induced Keto–Enol Tautomerization of DNA Base Molecules and Consequent [4 + 2]-like Cycloaddition on Si(111)7×7
Langmuir ( IF 3.7 ) Pub Date : 2022-01-12 , DOI: 10.1021/acs.langmuir.1c03173 Lei Zhang 1 , Hanieh Farkhondeh 1 , Fatemeh Rahnemaye Rahsepar 1, 2 , Avisek Chatterjee 1 , Kam Tong Leung 1
Langmuir ( IF 3.7 ) Pub Date : 2022-01-12 , DOI: 10.1021/acs.langmuir.1c03173 Lei Zhang 1 , Hanieh Farkhondeh 1 , Fatemeh Rahnemaye Rahsepar 1, 2 , Avisek Chatterjee 1 , Kam Tong Leung 1
Affiliation
|
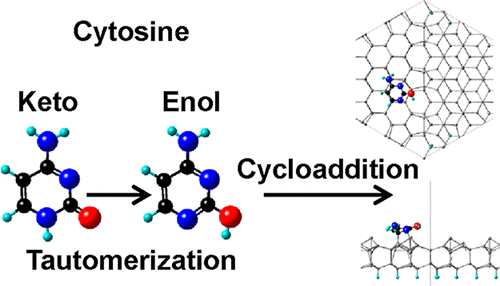
Adsorption and film growth of deoxyribonucleic acid (DNA) base molecules (cytosine, guanine, thymine, and adenine) on Si(111)7×7 have been studied by combining X-ray photoelectron spectroscopy (XPS) with ab initio calculations based on the density functional theory (DFT). Multiple tautomeric forms and keto–enol tautomerization are revealed by the O 1s, N 1s, and C 1s XPS spectra of the O-containing DNA bases: cytosine, guanine, and thymine. While the carbonyl group-containing keto tautomer is more stable in a thick film and in powder, the hydroxyl group-containing enol tautomer is found at the interface. The keto–enol tautomerization, as induced by the reactive Si(111)7×7 surface, leads to the formation of a conjugated aromatic six-membered ring with a delocalized π electron system and to the consequent [4 + 2]-like cycloaddition between the enol tautomer and the 7×7 surface. The DFT calculation suggests that the enol tautomer exhibits a kinetic advantage over the keto one for the [4 + 2]-like cycloaddition. Among the several plausible pathways for the cycloaddition provided by the enol tautomer, the experimentally determined one involves a ring N and ring C atom (a polar pair), rather than two ring C atoms (a nonpolar pair), to better match the polar Si adatom–restatom pair of the 7×7 surface. Furthermore, the reacted ring C atom does not have any attached terminal functional group (e.g., −NH2 and −OH). Further deposition leads to continuous film growth in the keto tautomeric form for cytosine and guanine. For the only O-free DNA base molecule, adenine, dative bonding N → Si, rather than the [4 + 2]-like cycloaddition, is observed on the 7×7 surface. Of the four DNA base molecules, adenine is also the only one with its aromaticity maintained when adsorbed on the Si(111)7×7 surface. A reactive surface like the 7×7 surface could therefore provide a new control to trigger tautomerization that is often associated with genetic mutation.
中文翻译:

表面诱导的 DNA 碱基分子的酮-烯醇互变异构和随后在 Si(111)7×7 上的 [4 + 2] 样环加成
通过将 X 射线光电子能谱 (XPS) 与基于密度泛函理论(DFT)。含 O 的 DNA 碱基:胞嘧啶、鸟嘌呤和胸腺嘧啶的 O 1s、N 1s 和 C 1s XPS 光谱揭示了多种互变异构形式和酮-烯醇互变异构。虽然含羰基的酮互变异构体在厚膜和粉末中更稳定,但含羟基的烯醇互变异构体存在于界面处。由反应性 Si(111)7×7 表面诱导的酮-烯醇互变异构化,导致形成具有离域 π 电子系统的共轭芳族六元环,并导致烯醇互变异构体和 7×7 表面之间的 [4 + 2] 类环加成反应。DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH2和-OH)。进一步的沉积导致胞嘧啶和鸟嘌呤的酮互变异构形式的连续薄膜生长。对于唯一的 O-free DNA 碱基分子,腺嘌呤,配位键 N → Si,而不是 [4 + 2] 样环加成,在 7×7 表面上观察到。在四种 DNA 碱基分子中,腺嘌呤也是唯一一种吸附在 Si(111)7×7 表面时仍保持芳香性的分子。因此,像 7×7 表面这样的反应性表面可以提供一种新的控制来触发通常与基因突变相关的互变异构化。
更新日期:2022-01-25
中文翻译:

表面诱导的 DNA 碱基分子的酮-烯醇互变异构和随后在 Si(111)7×7 上的 [4 + 2] 样环加成
通过将 X 射线光电子能谱 (XPS) 与基于密度泛函理论(DFT)。含 O 的 DNA 碱基:胞嘧啶、鸟嘌呤和胸腺嘧啶的 O 1s、N 1s 和 C 1s XPS 光谱揭示了多种互变异构形式和酮-烯醇互变异构。虽然含羰基的酮互变异构体在厚膜和粉末中更稳定,但含羟基的烯醇互变异构体存在于界面处。由反应性 Si(111)7×7 表面诱导的酮-烯醇互变异构化,导致形成具有离域 π 电子系统的共轭芳族六元环,并导致烯醇互变异构体和 7×7 表面之间的 [4 + 2] 类环加成反应。DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH DFT 计算表明烯醇互变异构体在 [4 + 2] 样环加成反应中表现出优于酮异构体的动力学优势。在由烯醇互变异构体提供的环加成的几种可能的途径中,实验确定的一种涉及环 N 和环 C 原子(极性对),而不是两个环 C 原子(非极性对),以更好地匹配极性 Si 7×7 表面的吸附原子-再原子对。此外,反应的环 C 原子没有任何连接的末端官能团(例如,-NH2和-OH)。进一步的沉积导致胞嘧啶和鸟嘌呤的酮互变异构形式的连续薄膜生长。对于唯一的 O-free DNA 碱基分子,腺嘌呤,配位键 N → Si,而不是 [4 + 2] 样环加成,在 7×7 表面上观察到。在四种 DNA 碱基分子中,腺嘌呤也是唯一一种吸附在 Si(111)7×7 表面时仍保持芳香性的分子。因此,像 7×7 表面这样的反应性表面可以提供一种新的控制来触发通常与基因突变相关的互变异构化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号