当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt-Catalyzed Intermolecular Hydroamination of Unactivated Alkenes Using NFSI as Nitrogen Source
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-01-13 , DOI: 10.1002/cjoc.202100827
Peng‐Wei Sun 1 , Ze Zhang 1 , Xinyao Wang 1 , Linshan Li 1 , Yuxin Li 1 , Zhengming Li 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-01-13 , DOI: 10.1002/cjoc.202100827
Peng‐Wei Sun 1 , Ze Zhang 1 , Xinyao Wang 1 , Linshan Li 1 , Yuxin Li 1 , Zhengming Li 1
Affiliation
|
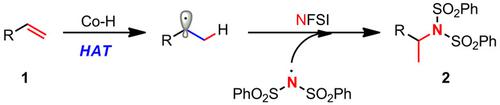
Cheap metal (Fe, Mn, and Co)-catalyzed hydroamination of alkenes has been an attractive method for synthesis of amines because of biocompatibility of metal, excellent Markovnikov selectivity and chemoselectivity. However, most reports are limited to unsaturated nitrogen sources (nitric oxide, azos, azides, cyano, etc.), for which aminated products are very limited. Notably, while used widely for fluorinating reaction, N-fluorobenzenesulfonimide (NFSI) as amine source for hydroamination has seldom been reported. Here we developed a cobalt-catalyzed intermolecular hydroamination of unactivated alkenes using NFSI as nitrogen source under mild conditions. The reaction exhibits excellent chemo- and regio-selectivity with no hydrofluorination or linear-selectivity products. Notably, the reaction proceeded with excellent yield even though the amount of Co(salen) catalyst was reduced to 0.2 mol%. Recently, a similar work was also reported by Zhang and coworkers (ref. 19).
中文翻译:

以 NFSI 为氮源的钴催化未活化烯烃的分子间加氢胺化
由于金属的生物相容性、优异的马尔科夫尼科夫选择性和化学选择性,廉价的金属(铁、锰和钴)催化的烯烃加氢胺化已成为一种有吸引力的胺合成方法。然而,大多数报道仅限于不饱和氮源(一氧化氮、偶氮、叠氮化物、氰基等),胺化产物非常有限。值得注意的是,虽然广泛用于氟化反应,但N-氟苯磺酰亚胺(NFSI)作为胺源用于加氢胺化的报道很少。在这里,我们开发了在温和条件下使用 NFSI 作为氮源的钴催化的未活化烯烃的分子间加氢胺化反应。该反应表现出优异的化学和区域选择性,没有氢氟化或线性选择性产物。值得注意的是,即使 Co(salen) 催化剂的量减少到 0.2 mol%,反应仍以优异的产率进行。最近,张和同事也报道了一项类似的工作(参考文献 19)。
更新日期:2022-01-13

中文翻译:

以 NFSI 为氮源的钴催化未活化烯烃的分子间加氢胺化
由于金属的生物相容性、优异的马尔科夫尼科夫选择性和化学选择性,廉价的金属(铁、锰和钴)催化的烯烃加氢胺化已成为一种有吸引力的胺合成方法。然而,大多数报道仅限于不饱和氮源(一氧化氮、偶氮、叠氮化物、氰基等),胺化产物非常有限。值得注意的是,虽然广泛用于氟化反应,但N-氟苯磺酰亚胺(NFSI)作为胺源用于加氢胺化的报道很少。在这里,我们开发了在温和条件下使用 NFSI 作为氮源的钴催化的未活化烯烃的分子间加氢胺化反应。该反应表现出优异的化学和区域选择性,没有氢氟化或线性选择性产物。值得注意的是,即使 Co(salen) 催化剂的量减少到 0.2 mol%,反应仍以优异的产率进行。最近,张和同事也报道了一项类似的工作(参考文献 19)。


































 京公网安备 11010802027423号
京公网安备 11010802027423号