Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2022-01-12 , DOI: 10.1016/j.jhazmat.2022.128261 Kaiyue Yin 1 , Juyuan Wang 2 , Sheng Zhai 1 , Xin Xu 1 , Tingting Li 1 , Shuchen Sun 1 , Shuai Xu 1 , Xuexue Zhang 1 , Cuiping Wang 3 , Yingshu Hao 1
|
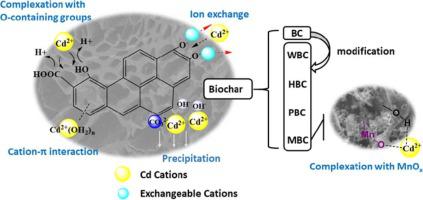
To understand the adsorption mechanisms of Cd2+ by oxidant-modified biochar (OMB) derived from Platanus orientalis Linn (POL) leaves, batch adsorption experiments and characterization were carried out. The results showed that, KMnO4-modified biochar (MBC) could more effectively remove Cd2+ from aqueous solution than H2O-, H2O2-, and K2Cr2O7-modified biochar (WBC, HBC and PBC, respectively). The highest removal efficiency was 98.57%, which was achieved by the addition of 2 g L−1 MBC at pH 6.0. According to the Langmuir fitting parameters, the maximum adsorption capacity for MBC was 52.5 mg g−1 at 30 ℃, which was twice as high as that for original biochar. MBC had the largest specific surface area with many particles distributed on the surface before and after adsorption, which were confirmed to be MnOx by XPS analysis. The complexation with MnOx was the main mechanism. Besides, O-containing groups complexation, precipitation, cation-π intraction, and ion exchange also participated in the adsorption. However, WBC, HBC and PBC did not achieve ideal removal effects, and their stability was inferior. This could be attributed to the weakening of ion exchange and precipitation. This study not only demonstrates the potential of MBC, but also provides insight into strategies for the utilization of waste resources.






























 京公网安备 11010802027423号
京公网安备 11010802027423号