当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphene Oxide–(Ferrocenylmethyl) Dimethylammonium Nitrate Composites as Catalysts for Ammonium Perchlorate Thermolysis
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2022-01-11 , DOI: 10.1021/acsanm.1c03817 Liping Jiang 1 , Xiaolong Fu 1 , Saiqin Meng 1 , Jizhen Li 1 , Wuxi Xie 1 , Xuezhong Fan 1
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2022-01-11 , DOI: 10.1021/acsanm.1c03817 Liping Jiang 1 , Xiaolong Fu 1 , Saiqin Meng 1 , Jizhen Li 1 , Wuxi Xie 1 , Xuezhong Fan 1
Affiliation
|
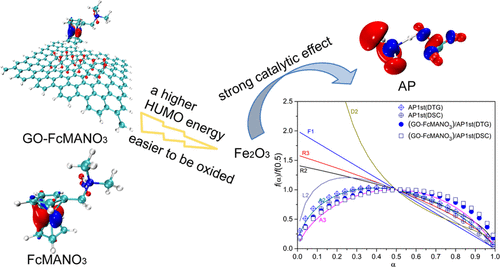
Graphene oxide has attracted attention due to its excellent catalytic properties, and it is expected to be used in the field of burning rate catalysis. To investigate the synergistic effect of graphene oxide and ferrocene derivatives, graphene oxide–(ferrocenylmethyl) dimethylammonium nitrate composites (GO–FcMANO3) were prepared and characterized by X-ray photoelectron spectroscopy (XPS), Raman analysis, X-ray diffraction (XRD) and scanning electron microscopy (SEM), and their thermal stability and catalytic effect on ammonium perchlorate (AP) were studied by thermogravimetry–differential scanning calorimetry (TG–DSC) techniques. Based on interaction region indicator (IRI) analysis, electrostatic potential (ESP) and energy decomposition analysis on the basis of forcefield (EDA-FF), the dispersion effect is the dominant component of interaction energy, and the contribution of electrostatic attraction is small. The frontier molecular orbitals illustrate that a higher highest occupied molecular orbital (HOMO) energy makes GO–FcMANO3 more susceptible to oxidization and is more conducive to catalyzing AP decomposition. The TG data illustrated that the presence of GO in the composites increased the thermal stability of FcMANO3 during AP decomposition. The catalytic effect of GO–FcMANO3 for AP decomposition manifested in two ways: it advanced the peak temperature in the second stage of AP decomposition and reduced the exothermic temperature width between two stages; it also increased the heat released during AP decomposition. Combined kinetic analysis and the Friedman method provided more detailed information about the catalytic behavior of composites for AP thermolysis, and GO–FcMANO3 composites decreased the Ea of the second stage of AP decomposition. The introduction of GO influenced the decomposition physical model of FcMANO3 for AP catalysis. The first stage of AP decomposition transformed from random nucleation and two-dimensional growth of nuclei model (A2) to random nucleation and three-dimensional growth of nuclei model (A3), and the second stage changed from the phase boundary-controlled reaction (R2) to random nucleation and two-dimensional growth of nuclei model (A2).
中文翻译:

氧化石墨烯-(二茂铁基甲基)二甲基硝酸铵复合材料作为高氯酸铵热解催化剂
氧化石墨烯因其优异的催化性能而备受关注,有望在燃速催化领域得到应用。为了研究氧化石墨烯和二茂铁衍生物的协同效应,氧化石墨烯-(二茂铁甲基)二甲基硝酸铵复合材料(GO-FcMANO 3)制备并采用X射线光电子能谱(XPS)、拉曼分析、X射线衍射(XRD)和扫描电子显微镜(SEM)对其进行表征,并通过热重分析研究了它们的热稳定性和对高氯酸铵(AP)的催化作用。 – 差示扫描量热法 (TG-DSC) 技术。基于相互作用区域指标(IRI)分析、静电势(ESP)和基于力场的能量分解分析(EDA-FF),色散效应是相互作用能的主要成分,静电引力的贡献很小。前沿分子轨道说明较高的最高占据分子轨道 (HOMO) 能量使 GO-FcMANO 3更容易被氧化,更有利于催化AP分解。TG 数据说明复合材料中 GO 的存在提高了 FcMANO 3在 AP 分解过程中的热稳定性。GO-FcMANO 3对 AP 分解的催化作用体现在两个方面:它提高了 AP 分解第二阶段的峰值温度,降低了两阶段之间的放热温度宽度;它还增加了 AP 分解过程中释放的热量。结合动力学分析和弗里德曼方法提供了关于复合材料对 AP 热解的催化行为的更详细信息,GO-FcMANO 3复合材料降低了E aAP分解的第二阶段。GO的引入影响了用于AP催化的FcMANO 3的分解物理模型。AP分解的第一阶段从随机成核和二维生长核模型(A2)转变为随机成核和三维生长核模型(A3),第二阶段从相界控制反应(R2)转变) 到原子核模型 (A2) 的随机成核和二维生长。
更新日期:2022-01-28
中文翻译:

氧化石墨烯-(二茂铁基甲基)二甲基硝酸铵复合材料作为高氯酸铵热解催化剂
氧化石墨烯因其优异的催化性能而备受关注,有望在燃速催化领域得到应用。为了研究氧化石墨烯和二茂铁衍生物的协同效应,氧化石墨烯-(二茂铁甲基)二甲基硝酸铵复合材料(GO-FcMANO 3)制备并采用X射线光电子能谱(XPS)、拉曼分析、X射线衍射(XRD)和扫描电子显微镜(SEM)对其进行表征,并通过热重分析研究了它们的热稳定性和对高氯酸铵(AP)的催化作用。 – 差示扫描量热法 (TG-DSC) 技术。基于相互作用区域指标(IRI)分析、静电势(ESP)和基于力场的能量分解分析(EDA-FF),色散效应是相互作用能的主要成分,静电引力的贡献很小。前沿分子轨道说明较高的最高占据分子轨道 (HOMO) 能量使 GO-FcMANO 3更容易被氧化,更有利于催化AP分解。TG 数据说明复合材料中 GO 的存在提高了 FcMANO 3在 AP 分解过程中的热稳定性。GO-FcMANO 3对 AP 分解的催化作用体现在两个方面:它提高了 AP 分解第二阶段的峰值温度,降低了两阶段之间的放热温度宽度;它还增加了 AP 分解过程中释放的热量。结合动力学分析和弗里德曼方法提供了关于复合材料对 AP 热解的催化行为的更详细信息,GO-FcMANO 3复合材料降低了E aAP分解的第二阶段。GO的引入影响了用于AP催化的FcMANO 3的分解物理模型。AP分解的第一阶段从随机成核和二维生长核模型(A2)转变为随机成核和三维生长核模型(A3),第二阶段从相界控制反应(R2)转变) 到原子核模型 (A2) 的随机成核和二维生长。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号