Synlett ( IF 1.7 ) Pub Date : 2022-01-10 , DOI: 10.1055/s-0041-1737325 Jean-François Soulé 1 , Zhuan Zhang 2 , Natacha Durand 1
|
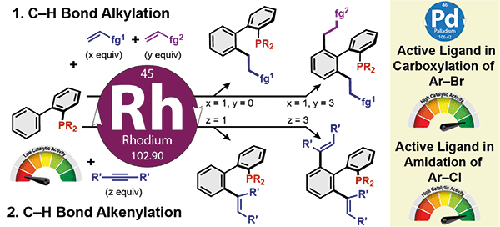
Trivalent-phosphorus-containing molecules are widely used in fields ranging from catalysis to materials science. Efficient catalytic methods for their modifications, providing straightforward access to novel hybrid structures with superior catalytic activities, are highly desired to facilitate reaction improvement or discovery. We have recently developed new methods for synthesizing polyfunctional phosphines by C–C cross-couplings through rhodium-catalyzed C–H bond activation. These methods use a native P(III) atom as a directing group, and can be used in regioselective late-stage functionalization of phosphine ligands. Interestingly, some of the modified phosphines outperform their parents in Pd-catalyzed cross-coupling reactions.
1 Introduction
2 Early Examples of Transition-Metal-Catalyzed P(III)-Directed C–H Bond Activation/Functionalizations
3 Synthesis of Polyfunctional Biarylphosphines by Late-Stage Alkylation: Application in Carboxylation Reactions
4 Synthesis of Polyfunctional Biarylphosphines by Late-Stage Alkenylation: Application in Amidation Reactions
5 Conclusion
中文翻译:

铑 (I) 催化的磷 (III) 导向 C-H 键官能化如何提高膦的催化活性
含三价磷的分子广泛用于从催化到材料科学的领域。非常需要对其修饰的有效催化方法,提供直接访问具有优异催化活性的新型杂化结构,以促进反应改进或发现。我们最近开发了通过铑催化的 C-H 键活化通过 C-C 交叉偶联合成多官能膦的新方法。这些方法使用天然 P(III) 原子作为导向基团,可用于膦配体的区域选择性后期官能化。有趣的是,一些改性膦在 Pd 催化的交叉偶联反应中的表现优于它们的亲本。
1 简介
2 过渡金属催化的 P(III) 导向 C-H 键活化/功能化的早期例子
3 后期烷基化合成多官能联芳基膦:在羧化反应中的应用
4 后期烯基化合成多官能联芳基膦:在酰胺化反应中的应用
5 结论











































 京公网安备 11010802027423号
京公网安备 11010802027423号