当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal and Catalytic Decomposition of 2-Hydroxyethylhydrazine and 2-Hydroxyethylhydrazinium Nitrate Ionic Liquid
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2022-01-11 , DOI: 10.1021/acs.jpca.1c07408
Steven D Chambreau 1 , Denisia M Popolan-Vaida 2, 3, 4 , Oleg Kostko 2 , Jae Kyoo Lee 5 , Zhenpeng Zhou 5 , Timothy A Brown 5 , Paul Jones 6 , Kuanliang Shao 6 , Jingsong Zhang 6 , Ghanshyam L Vaghjiani 7 , Richard N Zare 5 , Stephen R Leone 2, 8
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2022-01-11 , DOI: 10.1021/acs.jpca.1c07408
Steven D Chambreau 1 , Denisia M Popolan-Vaida 2, 3, 4 , Oleg Kostko 2 , Jae Kyoo Lee 5 , Zhenpeng Zhou 5 , Timothy A Brown 5 , Paul Jones 6 , Kuanliang Shao 6 , Jingsong Zhang 6 , Ghanshyam L Vaghjiani 7 , Richard N Zare 5 , Stephen R Leone 2, 8
Affiliation
|
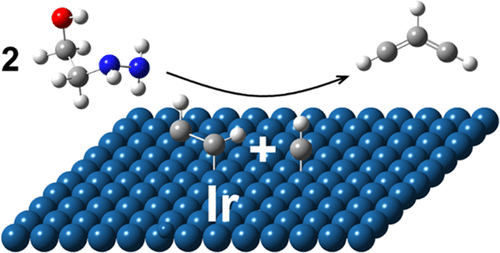
To develop chemical kinetics models for the combustion of ionic liquid-based monopropellants, identification of the elementary steps in the thermal and catalytic decomposition of components such as 2-hydroxyethylhydrazinium nitrate (HEHN) is needed but is currently not well understood. The first decomposition step in protic ionic liquids such as HEHN is typically the proton transfer from the cation to the anion, resulting in the formation of 2-hydroxyethylhydrazine (HEH) and HNO3. In the first part of this investigation, the high-temperature thermal decomposition of HEH is probed with flash pyrolysis (<1400 K) and vacuum ultraviolet (10.45 eV) photoionization time-of-flight mass spectrometry (VUV-PI-TOFMS). Next, the investigation into the thermal and catalytic decomposition of HEHN includes two mass spectrometric techniques: (1) tunable VUV-PI-TOFMS (7.4–15 eV) and (2) ambient ionization mass spectrometry utilizing both plasma and laser ionization techniques whereby HEHN is introduced onto a heated inert or iridium catalytic surface and the products are probed. The products can be identified by their masses, their ionization energies, and their collision-induced fragmentation patterns. Formation of product species indicates that catalytic surface recombination is an important reaction process in the decomposition mechanism of HEHN. The products and their possible elementary reaction mechanisms are discussed.
中文翻译:

2-羟乙基肼和2-羟乙基肼硝酸盐离子液体的热催化分解
为了开发基于离子液体的单推进剂燃烧的化学动力学模型,需要确定诸如 2-羟乙基硝酸肼 (HEHN) 等组分的热分解和催化分解的基本步骤,但目前尚不清楚。质子离子液体(如 HEHN)的第一个分解步骤通常是质子从阳离子转移到阴离子,从而形成 2-羟乙基肼 (HEH) 和 HNO 3. 在本研究的第一部分,HEH 的高温热分解通过闪蒸热解 (<1400 K) 和真空紫外 (10.45 eV) 光电离飞行时间质谱 (VUV-PI-TOFMS) 进行了探测。接下来,对 HEHN 的热分解和催化分解的研究包括两种质谱技术:(1)可调谐 VUV-PI-TOFMS(7.4-15 eV)和(2)利用等离子体和激光电离技术的环境电离质谱,其中 HEHN将其引入加热的惰性或铱催化表面,然后对产物进行探测。这些产物可以通过它们的质量、它们的电离能和它们的碰撞引起的碎裂模式来识别。产物种类的形成表明催化表面复合是HEHN分解机理中的一个重要反应过程。讨论了产物及其可能的基本反应机制。
更新日期:2022-01-27
中文翻译:

2-羟乙基肼和2-羟乙基肼硝酸盐离子液体的热催化分解
为了开发基于离子液体的单推进剂燃烧的化学动力学模型,需要确定诸如 2-羟乙基硝酸肼 (HEHN) 等组分的热分解和催化分解的基本步骤,但目前尚不清楚。质子离子液体(如 HEHN)的第一个分解步骤通常是质子从阳离子转移到阴离子,从而形成 2-羟乙基肼 (HEH) 和 HNO 3. 在本研究的第一部分,HEH 的高温热分解通过闪蒸热解 (<1400 K) 和真空紫外 (10.45 eV) 光电离飞行时间质谱 (VUV-PI-TOFMS) 进行了探测。接下来,对 HEHN 的热分解和催化分解的研究包括两种质谱技术:(1)可调谐 VUV-PI-TOFMS(7.4-15 eV)和(2)利用等离子体和激光电离技术的环境电离质谱,其中 HEHN将其引入加热的惰性或铱催化表面,然后对产物进行探测。这些产物可以通过它们的质量、它们的电离能和它们的碰撞引起的碎裂模式来识别。产物种类的形成表明催化表面复合是HEHN分解机理中的一个重要反应过程。讨论了产物及其可能的基本反应机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号